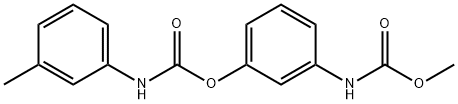
Phenmedipham synthesis
- Product Name:Phenmedipham
- CAS Number:13684-63-4
- Molecular formula:C16H16N2O4
- Molecular Weight:300.31
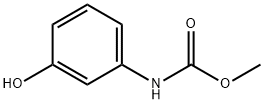
13683-89-1
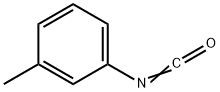
621-29-4
620-50-8

13684-63-4
Example 6: Preparation of methyl (3-(3-tolyl-carbamoyloxy)phenyl)carbamate Methyl N-(3-hydroxyphenyl)carbamate (8.35 g, 0.05 mol, prepared according to the method described in Example 1) was placed in a 150 mL beaker fitted with a stirrer, and water (75 mL) was added and stirred to mix. Borax (2.0 g) was added to the reaction system, followed by the slow dropwise addition of 3-tolyl isocyanate (5.3 g, 0.04 mol) through a dispensing funnel over a period of 45 minutes, with no need to adjust the temperature during the reaction. The pH was maintained at 9.2 during the reaction. after the dropwise addition, the reaction mixture was continued to be stirred for 1 hr. Upon completion of the reaction, the resulting finely suspended powdered product was filtered, washed with water and dried to afford the title compound (12.0 g, 100% yield, calculated on the basis of 3-tolyl isocyanate), melting point not determined.HPLC analysis showed the product to be 95.2% pure, with the remaining 4.8% consisting of unreacted N-(3-hydroxyphenyl)carbamic acid methyl ester and the by-products N,N'-di-3-tolylurea. -tolylurea.

13683-89-1
33 suppliers
inquiry

621-29-4
210 suppliers
$10.00/1g

620-50-8
5 suppliers
inquiry

13684-63-4
159 suppliers
$57.89/100mg
Yield:13684-63-4 100%
Reaction Conditions:
with borax in water
Steps:
6 Preparation of methyl (3-(3-tolyl-carbamoyloxy)phenyl)carbamate
EXAMPLE 6 Preparation of methyl (3-(3-tolyl-carbamoyloxy)phenyl)carbamate Methyl N-(3-hydroxyphenyl)carbamate (8.35 g, 0.05 mole) (prepared as described in Example 1) in water (75 ml) was placed in a 150 ml beaker equipped with a stirrer, and the mixture was stirred. Borax (2.0 g) was added, and over a period of 3/4 hour 3-tolyl isocyanate (5.3 g, 0.04 mole) was added by means of a separatory funnel without any adjustment of temperature. The pH value was 9.2 during the reaction time. After the addition of the 3-tolyl isocyanate, the stirring was continued for 1 hour. Subsequently the product, which was a very fine, suspended powder, was filtered, washed and dried to yield the title product (12.0 g, 100% calculated on the isocyanate intermediate), m.p. 139.8°-141.8° C. HPLC-Analysis showed a purity of 95.2%, the remaining 4.8% being methyl N-(3-hydroxyphenyl)carbamate and a by-product, N,N'-di-3-tolylurea.
References:
Kemisk Vaerk Koge A/S US4748265, 1988, A