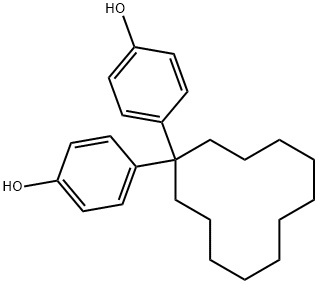
Phenol, 4,4'-cyclododecylidenebis- synthesis
- Product Name:Phenol, 4,4'-cyclododecylidenebis-
- CAS Number:29651-54-5
- Molecular formula:C24H32O2
- Molecular Weight:352.51
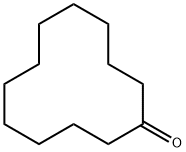
830-13-7

108-95-2

29651-54-5
In a 500 mL three-necked flask, 258.14 g (2.74 mol) of phenol and 13.73 g (0.137 mol) of 98% concentrated sulfuric acid were added sequentially, heated to 80 °C and maintained at this temperature with stirring for 1 hour. Upon completion of the reaction, the system was cooled to 60 °C, followed by the addition of 50.02 g (0.274 mol) cyclododecanone and 5.57 g (0.0275 mol) n-dodecylthiol. The reaction was stirred continuously for 8 hours at 64 °C to 68 °C under reduced pressure at 2.7 kPa while protected by nitrogen gas was passed. At the end of the reaction, the reaction mixture was allowed to stand overnight. After confirming the complete consumption of cyclododecanone by gas chromatography analysis, the reaction mixture was diluted with toluene and subsequently washed with ion-exchanged water and neutralized with sodium hydroxide solution. The organic layer was separated and the precipitated crystals were collected by filtration after cooling to room temperature. The crude product was recrystallized with a mixed solvent of methanol-water, filtered and dried to give 72.89 g of 1,1-bis(4-hydroxyphenyl)cyclododecane, which was detected by HPLC to be 100% pure in 75.4% yield.

830-13-7
171 suppliers
$22.00/25g

108-95-2
786 suppliers
$14.00/25g

29651-54-5
33 suppliers
$10.00/100mg
Yield:29651-54-5 77.04 g
Reaction Conditions:
Stage #1: phenolwith sulfuric acid at 80; for 1 h;
Stage #2: cyclododecanonewith 1-dodecylthiol at 30 - 65; under 9.75098 Torr; for 52 h;Reagent/catalyst;Temperature;
Steps:
1-6 Example 4
258.19 g (2.74 mol) of phenol and 13.72 g (0.137 mol) of 98% sulfuric acid were charged into a 500 ml glass reaction vessel equipped with a stirrer, a condenser and a thermometer, and the temperature was raised to 80 ° C. After stirring at the same temperature for 1 hour, 50.09 g (0.275 mol) of cyclododecanone at 60 ° C.,5.54 g (0.0274 mol) of n-dodecylmercaptan was added and reacted at 65 ° C. while removing water at 1.3 kPa until the remaining amount of cyclododecanone reached 50% of the initial charge amount.After recovering pressure to normal pressure with nitrogen gas, it was cooled to 30 ° C. and left to stand at the same temperature for 52 hours.0.15 g of the solidified reaction mass was scraped off, and the reaction mass was analyzed by gas chromatography. As a result, the remaining amount of cyclododecanone was 1% of the initially charged amount, and 1, 1-bis (4-hydroxy Phenyl) cyclododecane (the molar ratio to the initially charged amount of cyclododecanone) was 90.2%. Toluene and ion exchanged water were added to the obtained reaction mass, neutralized with sodium hydroxide, and then washed with ion exchanged water. The obtained organic layer was cooled as it was, the precipitated crystal was taken out by filtration and dried. The obtained crystals were recrystallized from methanol water, taken out by filtration and dried to obtain 77.04 g (0.219 mol, yield 79.5 mmol) of 1, 1-bis (4-hydroxyphenyl) cyclododecane %, HPLC purity 100%) was obtained
References:
JP2015/51935,2015,A Location in patent:Paragraph 0031-0040