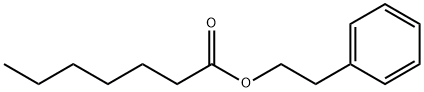
PHENYLETHYL-HEPTANOATE synthesis
- Product Name:PHENYLETHYL-HEPTANOATE
- CAS Number:5454-11-5
- Molecular formula:C15H22O2
- Molecular Weight:234.33
Yield:5454-11-5 81%
Reaction Conditions:
with triphenylphosphine;benzyl azide in neat (no solvent) at 140; for 0.166667 h;Microwave irradiation;
Steps:
General procedure for the synthesisof alkyl esters under heating condition (5a-p)
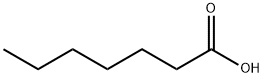
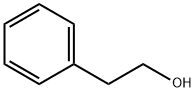
General procedure: Toa mixture of triphenylphosphine (1.0 mmol), benzyl azide (1.0 mmol) was addedslowly in a 2-phenylethanol (1.0 mmol) and carboxylic acid (drop wise manner toavoid accumulation of azide) and the mixture was place to microwave at 140° Cfor 10 minutes. (Caution As azides are potentially explosive, all the reactionsshould be carried out behind a blast shield with personal protective equipment.In particular, the sequence of additionof the reactants should be strictly followed to avoid the accumulation of organicazides. This has been achieved in thepresent investigation by the slow drop wise addition of the benzyl azide to thereaction mixture containing triphenylphosphine during which the azide group isinstantaneous converted to iminophosphorane and hence no difficulty wasencountered). After the completion ofthe reaction (as monitored by TLC), the mixture was poured onto crushed ice. Then the reaction mixture was extracted withdichloromethane and the organic layer was dried over anhydrous Na2SO4. The solvent was removed and the residue waspurified by column chromatography using silica gel as the adsorbent andpetroleum ether:ethyl acetate (98:2) as the mobile phase to afford thecorresponding carboxylic esters (5ap) as colourless oily liquids. Yield (73-85%).
References:
Dinesh, Murugan;Ranganathan, Raja;Archana, Sivasubramaniyan;Sathishkumar, Murugan;Roshan Banu, Mohamed Sulthan;Ponnuswamy, Alagusundaram [Synthetic Communications,2016,vol. 46,# 17,p. 1454 - 1460] Location in patent:supporting information