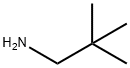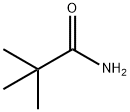
PIVALAMIDE synthesis
- Product Name:PIVALAMIDE
- CAS Number:754-10-9
- Molecular formula:C5H11NO
- Molecular Weight:101.15
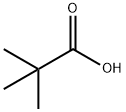
75-98-9

754-10-9
The general procedure for the synthesis of trimethylacetamide from pivalic acid is as follows: to a 10 mL THF colorless solution containing 75 mg (0.50 mmol) of 3-phenylpropionic acid 1a at 0 °C, 67 μL (0.70 mmol, 1.4 eq.) of ethyl chloroformate and 209 μL (1.5 mmol, 3.0 eq.) of triethylamine were added sequentially. The reaction mixture was stirred at 0 °C for 30 min before adding 0.75 mL of 1.0 M NH4Cl aqueous solution (0.75 mmol, 1.5 equiv). Stirring was continued at 0 °C for 30 min, followed by the addition of 5 mL of water. The reaction mixture was extracted with 30 mL of ethyl acetate and the aqueous layer was then extracted with 20 mL of ethyl acetate. The organic layers were combined, washed with 5 mL of brine and dried with anhydrous magnesium sulfate. The crude product was purified by silica gel column chromatography (eluent: ethyl acetate) to give 72 mg (96% yield) of 3-phenylpropionamide 2a. Synthesis of trimethylacetamide 2d: 44 mg (87% yield) of a colorless solid was obtained; melting point: 105-108 °C; 1H NMR (400 MHz, CDCl3): δ1.23 (s, 9H, CH3*3), δ1.21 (br, 1H, 1H). 5.21 (br, 1H, NHA), 5.59 (br, 1H, NHB); 13C NMR (100MHz, CDCl3): δ 27.7,38.7,181.6; IR (KBr, vmax/cm-1): 3398 (CONH), 3205 (CONH), 2960 (CH3), 1653 (CON), 1653 (CON). 1624 (CON); HRMS (ESI-TOF): calculated value C5H11NONa(M+Na)+: 124.0733, measured value: 124.0723.
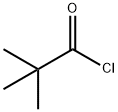
3282-30-2
375 suppliers
$18.00/100ml
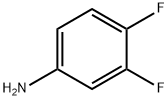
3863-11-4
340 suppliers
$10.00/25 g

754-10-9
175 suppliers
$7.00/5g
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane
Steps:
13.A Part A:
Part A: Preparation of N-trimethylacetyl-3,4-difluoroandlide. To a solution of 3,4-difluoroaniline (19 mL, 191 mmol) in methylene chloride (500 mL) at 0° was added triethylamine (32 mL, 230 mmol) followed dropwise with trimethylacetyl chloride (24 mL, 191 mmol) and the resulting reaction mixture was allowed to stir at room temperature for 3 h. The reaction mixture was poured onto 3N HCl ard extracted with methylene chloride (3*100 mL) and the combined organic extracts were dried over anhydrous NaSO4 and concentrated in vacuo. The residue was taken up in hexanes (300 mL) and filtered through a sintered glass funnel. The solids are washed thoroughly with hexanes (500 mL) and dried under vacuum to give 37.36 g of the pivaloyl amide as a solic (40.68 g theoretical, 92% yield).
References:
Dupont Pharmaceuticals Company US6140320, 2000, A

75-98-9
430 suppliers
$10.00/10g

754-10-9
175 suppliers
$7.00/5g
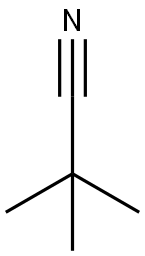
630-18-2
196 suppliers
$10.00/1g

754-10-9
175 suppliers
$7.00/5g

3282-30-2
375 suppliers
$18.00/100ml

754-10-9
175 suppliers
$7.00/5g