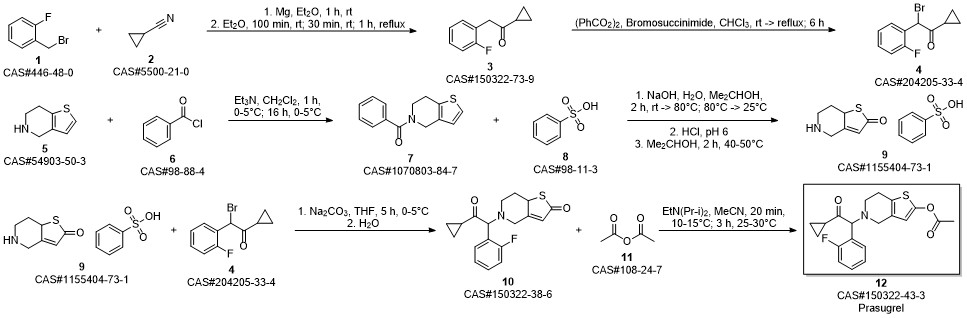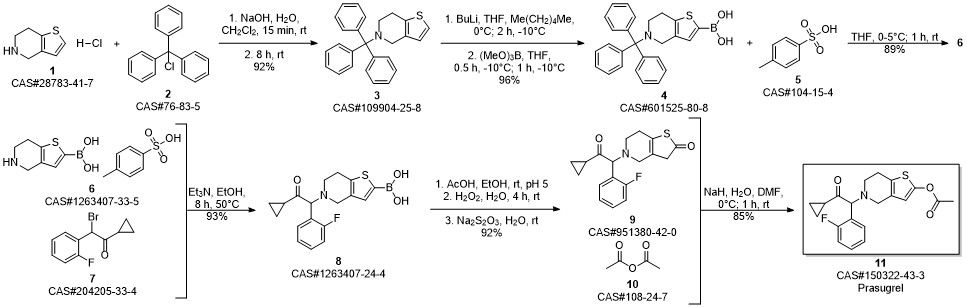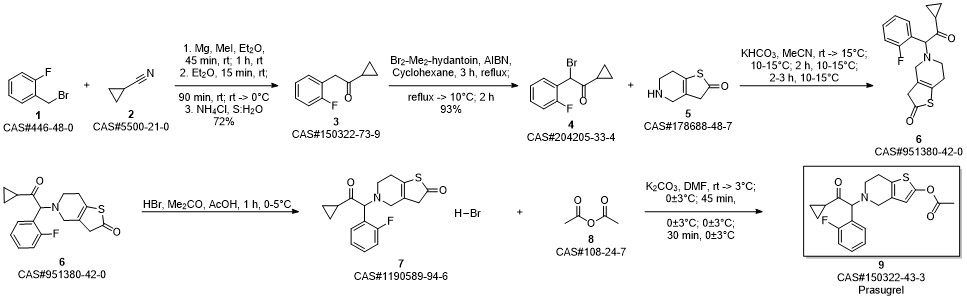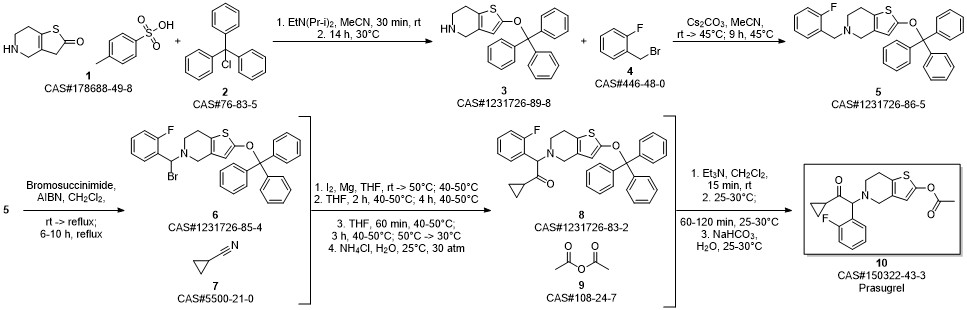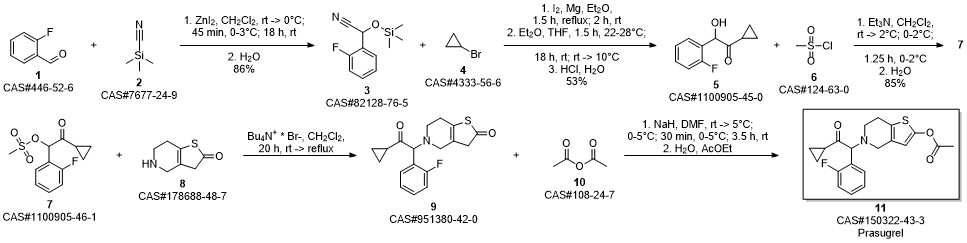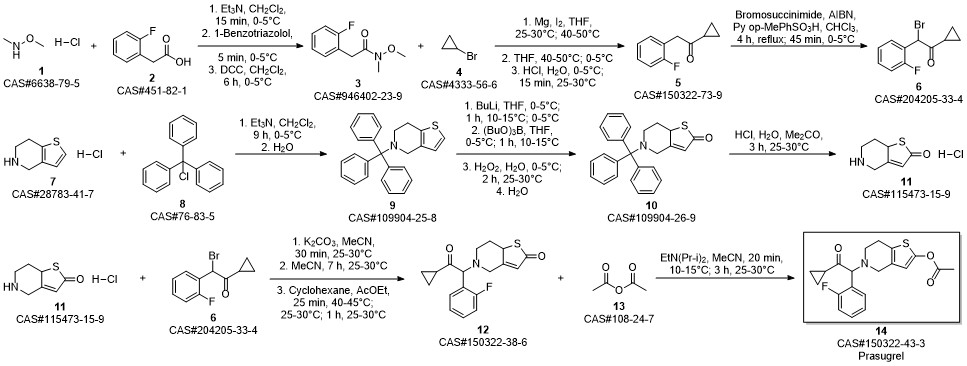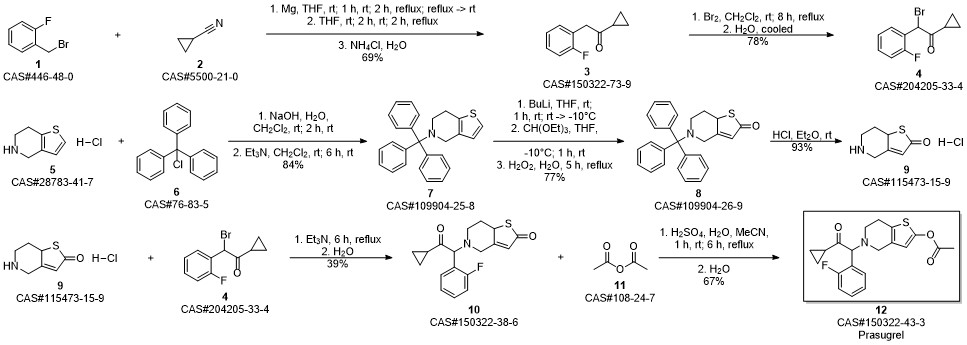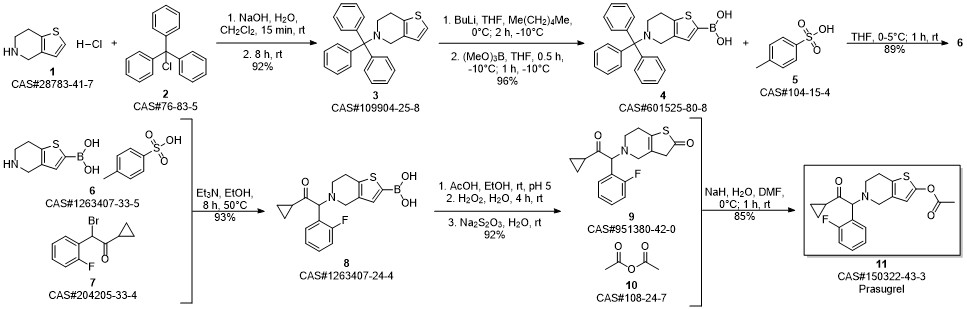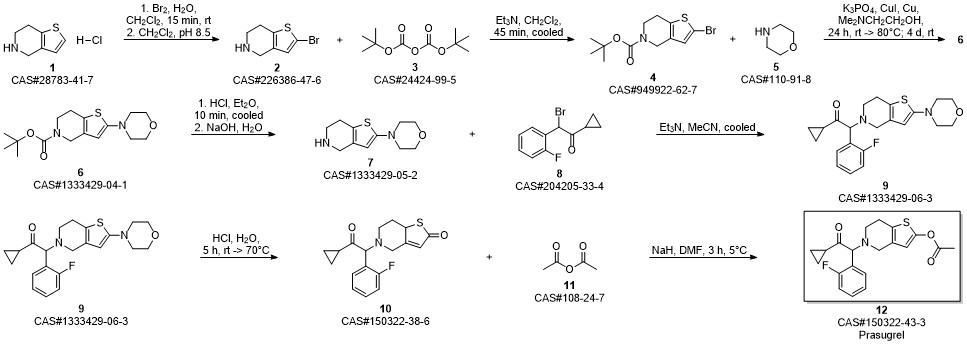
Prasugrel synthesis
- Product Name:Prasugrel
- CAS Number:150322-43-3
- Molecular formula:C20H20FNO3S
- Molecular Weight:373.44
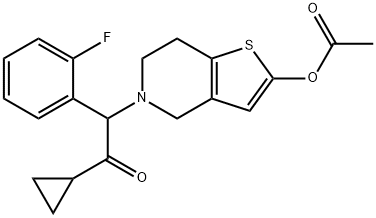
Padi, Pratap Reddy; Peri, Seetha Rama Sarma; Ganta, Madhusudhan Reddy; Polavarapu, Srinivas; Cherukupally, Praveen; Ireni, Babu; Padamata, Shailaja; Jonnada, Krishna; Vinigari, Krishna; Nerella, Kavitha. A process for preparing prasugrel and its salts and polymorphs. Assignee Dr. Reddy's Laboratories Ltd., India; Dr. Reddy's Laboratories, Inc. WO 2009062044. (2009).
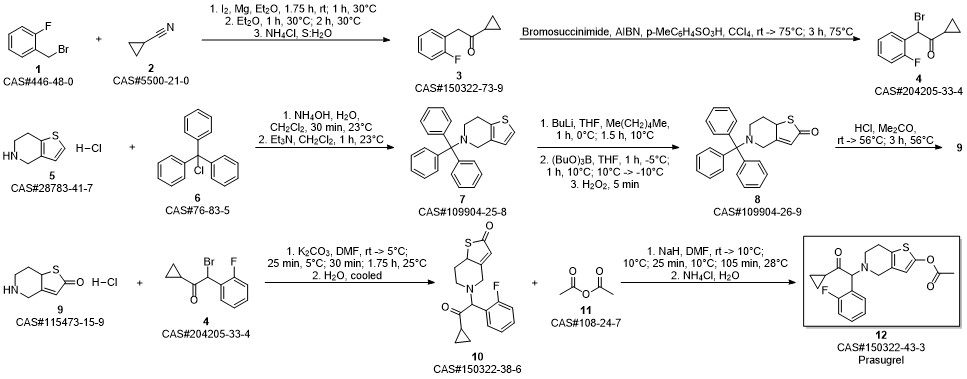
![5-[2-Cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one](/CAS/GIF/150322-38-6.gif)
150322-38-6
129 suppliers
$158.08/250mg

75-36-5
579 suppliers
$17.92/100G

150322-43-3
361 suppliers
$8.00/25mg
Yield:150322-43-3 99%
Reaction Conditions:
Stage #1:5-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one;acetyl chloride with acetic acid in toluene at 20;Inert atmosphere;
Stage #2: with sodium hydrogencarbonate in water;toluene
Steps:
12.B
Method BIn a 2 ltr 4-necked flask equipped with a thermometer and mechanical stirrer, 5-(a- cyclopropyIcarbonyl-2-fluoro-benzyl)-4,5,6,7-tetrahydrothieno -[3,2-c]-pyridine (100 g, 0.30 moles) was suspended in a mixture of toluene (400ml) and acetic acid (100 ml) under nitrogen atmosphere at 20 +/-5 °C and stir for 10 to 20 min. Slowly add acetyl chloride (180 g, 2.29 moles) in reaction mass at 20 +/-2 °C and stir for 12 to 16 hrs at 20 +/-2 °C. Quench the reaction mass into 30% sodium bicarbonate solution. Product extract with ethyl acetate (2 x 500 ml), organic layer washed with sodium chloride solution then water and dried over sodium sulfate. Recover the solvent under vacuum and crystalise the product in methanol, ethyl acetate, hexane or mixture thereof. Filter the product and slurry wash with methanol or ethyl acetate or hexane or mixture thereof. Dry the material at atmospheric pressure at 40-45 °C to obtain prasugrel (70 g, 99%). Method AIn a 2 ltr 4-necked flask equipped with a thermometer and mechanical stirrer, 2-(tert- Butyldimethylsilyloxy)-5-(a-cyclopropylcarbonyl-2-fluoro-benzyl)-4,5,6,7- tetrahydrothieno -[3,2-c]-pyridine (100 g, 0.22 moles) was suspended in a mixture of toluene (400ml) and acetic acid (100 ml) under nitrogen atmosphere at 20 +/-5 °C and stir for 10 to 20 min. Slowly add a solution of acetyl chloride (30 g, 0.38 moles) in acetic acid (140 ml) at 20 +/-2 °C. Stir the reaction mass for 4 to 6 hrs at 20 +/-2 °C. Slowly add acetyl chloride (180 g, 2.29 moles) in reaction mass at at 20 +/-2 °C and stir for 12 to 16 hrs at 20 +/-2 °C. Quench the reaction mass into 30% sodium bicarbonate solution. Product extract with ethyl acetate (2 x 500 ml), organic layer washed with sodium chloride solution then water and dried over sodium sulfate. Recover the solvent under vacuum and crystalise the product in methanol, ethyl acetate, hexane or mixture thereof. Filter the product and slurry wash with methanol or ethyl acetate or hexane or mixture thereof. Dry the material at atmospheric pressure at 40-45 °C to obtain prasugrel (80 g, 99%).
References:
MAYUKA LABS PVT. LTD.;KRISHNAMURTHY, Chandra, Sekhar, Nakka;SINGH, Jagat;KHAN, Mohd, Yunus WO2012/1486, 2012, A1 Location in patent:Page/Page column 23
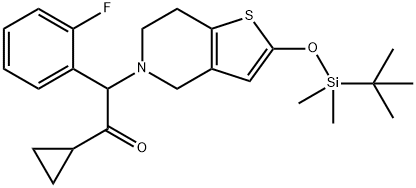
952340-38-4
11 suppliers
$165.00/10mg

108-24-7
0 suppliers
$14.00/250ML

150322-43-3
361 suppliers
$8.00/25mg
![5-[2-Cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one](/CAS/GIF/150322-38-6.gif)
150322-38-6
129 suppliers
$158.08/250mg

150322-43-3
361 suppliers
$8.00/25mg
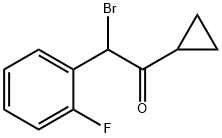
204205-33-4
243 suppliers
$30.00/5g
![5,6,7,7a-Tetrahydrothieno[3,2-c]pyridin-2(4H)-one 4-methylbenzenesulfonate](/CAS/GIF/952340-39-5.gif)
952340-39-5
65 suppliers
$309.00/250mg

108-24-7
0 suppliers
$14.00/250ML

150322-43-3
361 suppliers
$8.00/25mg
![5-[2-Cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one](/CAS/GIF/150322-38-6.gif)
150322-38-6
129 suppliers
$158.08/250mg

108-24-7
0 suppliers
$14.00/250ML

150322-43-3
361 suppliers
$8.00/25mg