primaperone synthesis
- Product Name:primaperone
- CAS Number:1219-35-8
- Molecular formula:C15H20FNO
- Molecular Weight:0
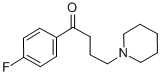
![1-[3-(p-fluorobenzoyl)propyl]piperidinium chloride](/CAS/GIF/1219-36-9.gif)
1219-36-9
3 suppliers
inquiry

1219-35-8
6 suppliers
inquiry
Yield:1219-35-8 371.7 g
Reaction Conditions:
with sodium hydroxide in water; pH=12 - 14; for 0.5 h;
Steps:
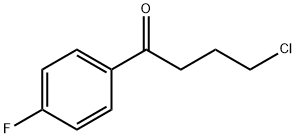
1.3 (3) Intermediate-1 free base: 1-(4-fluorophenyl)-4-(piperidin-1-yl)butan-1-one
(3) Intermediate-1 free base: 1-(4-fluorophenyl)-4-(piperidin-1-yl)butan-1-one. In a 4 L Erlenmeyer flask, 428 g (1.50 mol) of of 1-(4-fluorophenyl)-4-(piperidin-1-yl)butan-1-one hydrochloride was dissolved in 1.7 L of water. 3N NaOH (577 mL; 1.73 mol; 1.15 eq.) was added to the flask to reach a pH between 12-14. The mixture was vigorously stirred for 30 minutes. The oily product was extracted with dichloromethane (2 x 770 mL). The phases were let to separate and the organic phase was washed with water (2 x 380 mL). The phases were again separated and the organic phase was dried over anhydrous Na2SO4 for 1 h. The suspension was vacuum filtered and the filtrate concentrated on rotatory evaporator. The oily residue was dried in an oven at 50°C for 12 h. After complete drying, the material was cooled to obtain 371.7 g of 1-(4-fluorophenyl)-4-(piperidin-1-yl)butan-1-one free base, as an amorphous solid and unctuous to the touch.MS: m/z (rel. intensity) = 249 [M]+, 123, 111, 98 (100%).
References:
EP2905278,2015,A1 Location in patent:Paragraph 0152
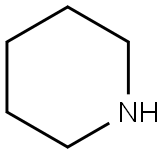
110-89-4
3 suppliers
$15.96/100ML
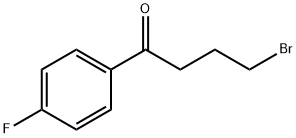
40132-01-2
27 suppliers
$140.00/100mg

1219-35-8
6 suppliers
inquiry