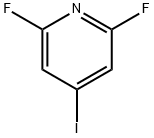
PYRIDINE, 2,6-DIFLUORO-4-IODO- synthesis
- Product Name:PYRIDINE, 2,6-DIFLUORO-4-IODO-
- CAS Number:685517-71-9
- Molecular formula:C5H2F2IN
- Molecular Weight:240.98
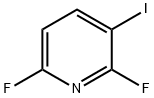
685517-67-3

685517-71-9
The general procedure for the synthesis of 2,6-difluoro-4-iodopyridine from 2,6-difluoro-3-iodopyridine was as follows: n-butyllithium (1.66 mL, 4.15 mmol, 2.5 mol/L hexane solution) was slowly added dropwise to a solution of tetrahydrofuran (THF, 2 mL) with diisopropylamine (1.30 mL, 4.15 mmol) at -78 °C. 25 minutes later, a solution of 2,6-difluoro-3-iodopyridine (1.00 g, 4.15 mmol) in THF (5 mL) was added dropwise to the above mixture via cannula. The reaction mixture was kept at -78 °C for 12 h. THF (2 mL) solution in water (0.2 mL, 11 mmol) was subsequently added and slowly warmed up to room temperature over a period of 1 h. The reaction was carried out at -78 °C for 1 h. The reaction was carried out in a controlled manner. Upon completion of the reaction, the reaction mixture was washed with 10% aqueous Na2SO3 solution (5 mL) and extracted with ethyl acetate (3 x 50 mL). The organic phases were combined and dried over anhydrous magnesium sulfate (MgSO4) and subsequently concentrated under reduced pressure. The target product 2,6-difluoro-4-iodopyridine was obtained by hexane crystallization as colorless crystals (740 mg, 74% yield). The product was characterized as follows: 1H NMR (400 MHz, CDCl3): δ 7.22 (t, 2H, J = 1.2 Hz); 13C NMR (100 MHz, CDCl3): δ 161.1 (dd, J = 250, 16 Hz, 2C), 115.6 (dd, J = 24, 10 Hz, 2C), 110.3; 19F NMR (376 MHz, CDCl3): δ -68.1; GC-MS m/z: Calculated value 241 [M+], measured value 241; Melting point: 78-80 °C.

685517-67-3
96 suppliers
$16.00/1g

685517-71-9
114 suppliers
$22.00/250mg
Yield:685517-71-9 74%
Reaction Conditions:
Stage #1:2,6-difluoro-3-iodopyridine with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 12 h;
Stage #2: with water in tetrahydrofuran;hexane at -78 - 20; for 1 h;
Steps:
4.1.2 DFIDP
n-Butyllithium (1.66mL, 4.15mmol, 2.5mol/L in hexane) was added dropwise to a solution of diisopropylamine (1.30mL, 4.15mmol) in THF (2mL) at -78°C. After 25min 2,6-difluoro-3-iodopyridine (1.00g, 4.15mmol) in THF (5mL) was added dropwise into this mixture by cannula. After 12h at -78°C, H2O (0.2mL, 11mmol) in THF (2mL) was added and the mixture was warmed to room temperature during 1h. The mixture was washed with 10% aqueous Na2SO3 (5mL) and extracted with ethyl acetate (3×50mL). The organic extracts were dried (MgSO4) and concentrated in vacuo. Crystallization from hexane afforded the product as colorless crystals (740mg, 74%). 1H NMR (400MHz, CDCl3): δ 7.22 (t, 2H,J=1.2). 13C NMR (100MHz, CDCl3): 161.1 (dd, J=250, 16, 2C), 115.6 (dd, J=24, 10, 2C), 110.3. 19F NMR (376MHz, CDCl3): δ -68.1. GC-MS m/z calcd. for C5H2F2IN [M+]: 241, found: 241. MP: 78-80°C.
References:
Jin, Xin;Cramer, Jacob R.;Chen, Qi-Wei;Liang, Hai-Lin;Shang, Jian;Shao, Xiang;Chen, Wei;Xu, Guo-Qin;Gothelf, Kurt V.;Wu, Kai [Chinese Chemical Letters,2017,vol. 28,# 3,p. 525 - 530]

685517-67-3
96 suppliers
$16.00/1g

685517-71-9
114 suppliers
$22.00/250mg
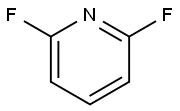
1513-65-1
344 suppliers
$6.00/5g

1513-65-1
344 suppliers
$6.00/5g

685517-71-9
114 suppliers
$22.00/250mg