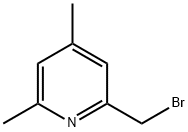
Pyridine, 2-(bromomethyl)-4,6-dimethyl- (9CI) synthesis
- Product Name:Pyridine, 2-(bromomethyl)-4,6-dimethyl- (9CI)
- CAS Number:79313-01-2
- Molecular formula:C8H10BrN
- Molecular Weight:200.08
108-75-8
456 suppliers
$5.00/10g

79313-01-2
37 suppliers
$60.50/50 mg
Yield: 42.5%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane at 90; for 16 h;
Steps:
Scheme 13. 2-(bromomethyl)-4,6-dimethylpyridine
Scheme 13. 2-(bromomethyl)-4,6-dimethylpyridine Z56 Z57 To a stirred solution of 2,4,6-trimethylpyridine (1.0 g, 8.26 mmol, 1.0 equiv.) in carbon tetrachloride (15 ml_) was added NBS (1.46 g, 8.26 mmol, 1.0 equiv.) followed by dibenzoyl peroxide (0.5 g, 2.06 mmol, 0.25 equiv.). The reaction mixture was heated to 90 °C for 16 h. The reaction mixture was cooled to room temperature and evaporated to obtain crude compound. The crude product was purified over silica gel flash column chromatography. The compound eluted out in 15 % EtOAc: Hexanes. The pure fractions were evaporated to obtain 2-(bromomethyl)-4,6-dimethylpyridine (0.7 g, 42.5%) as an orange oil. LCMS (ES) m/z = 200.0, 202.0 [M+H]+. H NMR (400 MHz, DMSO-d6) δ ppm 2.27 (s, 3H), 2.39 (s, 3H), 4.57 (s, 2H), 7.0 (s, 1 H), 7.15 (s, 1 H).
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO.2) LIMITED;AXTEN, Jeffrey Michael;FAUCHER, Nicolas Eric;DAUGAN, Alain Claude-Marie WO2017/46739, 2017, A1 Location in patent:Page/Page column 54; 55