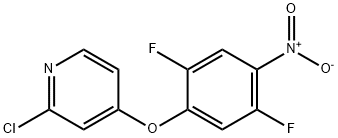
Pyridine,2-chloro-4-(2,5-difluoro-4-nitrophenoxy)- synthesis
- Product Name:Pyridine,2-chloro-4-(2,5-difluoro-4-nitrophenoxy)-
- CAS Number:1225278-64-7
- Molecular formula:C11H5ClF2N2O3
- Molecular Weight:286.62
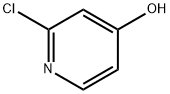
17368-12-6
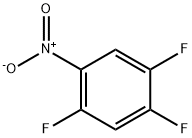
2105-61-5

1225278-64-7
Sodium hydride (136 mg, 3.4 mmol, 60% mineral oil dispersion) was added batchwise to an anhydrous N,N-dimethylformamide (DMF, 38 mL) solution of 2-chloro-4-hydroxypyridine (2 g, 15.4 mmol) under argon protection. The reaction mixture was stirred at 0 °C for 1 hour. Subsequently, a solution of 2,4,5-trifluoronitrobenzene (626 mg, 3.1 mmol) in anhydrous DMF (7.6 mL) was added dropwise and the reaction system was warmed up to 90 °C under argon protection with continuous stirring for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature and stirring was continued overnight. The solvent was removed by distillation under reduced pressure and the crude product was partitioned with 50 mL of water and 50 mL of ethyl acetate (EtOAc). The aqueous phase was extracted with ethyl acetate (3 x 50 mL) and the combined organic phases were washed with saturated saline and dried over anhydrous sodium sulfate. After concentration under reduced pressure, the target compound 2-chloro-4-(2,5-difluoro-4-nitrophenoxy)pyridine (3.57 g, 81% yield) was purified by silica gel column chromatography (eluent: hexane/ethyl acetate gradient). The product was characterized by 1H NMR (400 MHz, DMSO-d6) and mass spectrometry (ESI): 1H NMR δ 8.43-8.33 (m, 2H), 7.85-7.79 (m, 1H), 7.33 (d, 1H), 7.20-7.18 (m, 1H); MS (ESI) m/z: 287.0 [M+H]+.

17368-12-6
284 suppliers
$4.00/1g

2105-61-5
216 suppliers
$6.00/5g

1225278-64-7
37 suppliers
inquiry
Yield:1225278-64-7 81%
Reaction Conditions:
Stage #1: 2-chloropyridin-4-olwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 1 h;Inert atmosphere;
Stage #2: 1,2,4-trifluoro-5-nitrobenzene in N,N-dimethyl-formamide;mineral oil at 90; for 3 h;Product distribution / selectivity;Inert atmosphere;
Steps:
A.15
Sodium hydride (136 mg, 3.4 mmol, 60% in mineral) was added to a O°C solution of 2-chloropyridin-4-ol (2 g, 15.4 mmol) in DMF (38 mL) under Ar. The mixture was stirred at 0 °C for 1 h. A solution of 1,2,4-trifluoro-5-nitrobenzene (626 mg, 3.1 mmol) in DMF (7.6 ml) was added and the reaction was stirred under Ar at 90 °C for 3 h. The mixture was cooled to RT and stirred overnight. The solvent was removed under reduced pressure and the crude product was partitioned between water (50 ml) and EtOAc (50 ml). The mixture was extracted with EtOAc (3 x 50 ml). The combined organic extracts were washed with brine, dried (Na2SO4), concentrated under reduced pressure and purified by silica gel column chromatography (hexanes/EtOAc) to yield 2-chloro-4-(2,5-difluoro-4-nitrophenoxy)pyridine (3.57 g, 81% yield). 1H NMR (400 MHz, DMSO-d6): δ 8.43-8.33 (m, 2H), 7.85-7.79 (m, 1 H), 7.33 (d, 1H), 7.20-7.18 (m , 1H); MS (ESI) m/z: 287.0 (M+H+).
References:
WO2010/51373,2010,A1 Location in patent:Page/Page column 73-74