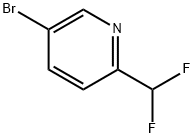
PYRIDINE, 5-BROMO-2-(DIFLUOROMETHYL)- synthesis
- Product Name:PYRIDINE, 5-BROMO-2-(DIFLUOROMETHYL)-
- CAS Number:845827-13-6
- Molecular formula:C6H4BrF2N
- Molecular Weight:208
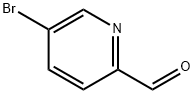
31181-90-5

845827-13-6
General procedure for the synthesis of 5-bromo-2-difluoromethylpyridine from 5-bromo-2-pyridinecarboxaldehyde: To a cooled solution of 5-bromopyridine-2-carboxaldehyde (A) (7.0 g, 38 mmol) in dichloromethane (300 mL) was slowly added diethylaminosulfur trifluoride (DAST, 10.8 mL, 83 mmol) at -78 °C. The reaction mixture was gradually warmed to room temperature over a period of 6 h. The reaction was subsequently quenched by the slow addition of water. The reaction mixture was washed with saturated aqueous sodium bicarbonate solution and dried with anhydrous sodium sulfate. Concentration and purification by silica gel column chromatography (dichloromethane as eluent) afforded 5-bromo-2-difluoromethylpyridine (B) as brown crystals (5.3 g, 67% yield).1H NMR (300 MHz, CDCl3) δ 8.8 (s, 1H), 8.0 (d, 1H), 7.6 (d, 1H), 6.6 (t, 1H).
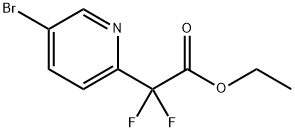
294181-95-6
49 suppliers
$55.00/100mg

845827-13-6
90 suppliers
$30.00/250 mg
Yield: 40%
Reaction Conditions:
with potassium fluoride in water;N,N-dimethyl-formamide at 170; for 24 h;Schlenk technique;Inert atmosphere;
Steps:
1
According to the following procedure,5-Bromo-2-difluoromethylpyridine (Compound 4a) was prepared. In the Schlenk tube,The above-obtained 2- (5-bromopyridin-2-yl) -2,2-difluoroacetic acid ethyl ester (compound 3a) (224.1 mg, 0.8 mmol)Potassium fluoride (232.4 mg, 4.0 mmol) and water (72.1 mg, 4.0 mmol) were added and the mixture was stirred at 170 ° C. for 24 hours in a DMF solvent (3.2 mL) under a nitrogen atmosphere. After the reaction, 2,2,2-trifluoroethanol was added as an internal standard, As a result of 19 F-NMR measurement, it was found that 5-bromo-2-difluoromethylpyridine (compound 4a) was produced in a yield of 89%.After that, extraction with diethyl ether, washing with water,And dried over anhydrous sodium sulfate.Anhydrous sodium sulfate was removed by filtration,The filtrate was concentrated under reduced pressure.The residue was purified by silica gel column chromatography (Hexane: diethylether = 20: 1) 5-Bromo-2-difluoromethylpyridine (Compound 4a) (66.8 mg, 0.32 mmol, yield: 40%) was obtained.
References:
GUNMA UNIVERSITY;CENTRAL GLASS COMPANY LIMITED;AMII, HIDEKI;FUJIKAWA, KENICHI;MATSUURA, MAKOTO JP5679855, 2015, B2 Location in patent:Paragraph 0082-0084

31181-90-5
359 suppliers
$5.00/250mg

845827-13-6
90 suppliers
$30.00/250 mg
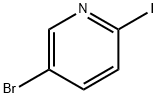
223463-13-6
382 suppliers
$5.00/1g
1438388-96-5
0 suppliers
inquiry

845827-13-6
90 suppliers
$30.00/250 mg

223463-13-6
382 suppliers
$5.00/1g

845827-13-6
90 suppliers
$30.00/250 mg