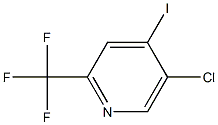
Pyridine, 5-chloro-4-iodo-2-(trifluoroMethyl)- synthesis
- Product Name:Pyridine, 5-chloro-4-iodo-2-(trifluoroMethyl)-
- CAS Number:823221-95-0
- Molecular formula:C6H2ClF3IN
- Molecular Weight:307.4395
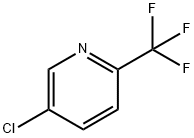
349-94-0
118 suppliers
$13.00/1g

823221-95-0
49 suppliers
$119.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 5-chloro-2-(trifluoromethyl)pyridinewith iodine;lithium diisopropyl amide in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2: with iodine in tetrahydrofuran at -78; for 2 h;
Steps:
104.C 5-chloro-4-iodo-2-(trifluoromethyl)pyridine
Step C 5-chloro-4-iodo-2-(trifluoromethyl)pyridine Into a 100-mL 3-necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen, was placed a solution of 5-chloro-2-(trifluoromethyl)pyridine (as prepared in the previous step, 5 g, 27.62 mmol, 1.00 equiv) in tetrahydrofuran (50 mL). LDA (3 g, 28.04 mmol, 1.05 equiv, as a THF solution) was added dropwise with stirring at -78° C. The resulting solution was stirred for 30 min at -78° C. A solution of I2 (7.4 g, 29.13 mmol, 1.05 equiv) in tetrahydrofuran (10 mL) was added dropwise with stirring at -78° C. The reaction mixture was stirred for an additional 2 h at -78° C., quenched with 15 mL of Na2S2O3(1M) and diluted with 100 mL of water. The resulting mixture was extracted with 3*50 mL of ether. The combined organic layers were washed with 50 ml brine, dried (Na2SO4), and concentrated under vacuum. The residue was purified by chromatography over a silica gel column with ethyl acetate/petroleum ether (0:1), yielding 5-chloro-4-iodo-2-(trifluoromethyl)pyridine as a white solid.
References:
US2011/306592,2011,A1 Location in patent:Page/Page column 92
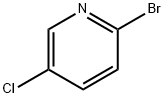
40473-01-6
426 suppliers
$5.00/1g

823221-95-0
49 suppliers
$119.00/100mg
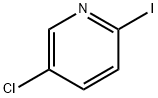
244221-57-6
179 suppliers
$13.43/250mgs:

823221-95-0
49 suppliers
$119.00/100mg