Pyrrolidine, 1-bicyclo[2.2.1]hept-2-yl- (9CI) synthesis
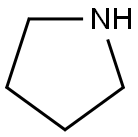
- Product Name:Pyrrolidine, 1-bicyclo[2.2.1]hept-2-yl- (9CI)
- CAS Number:168031-65-0
- Molecular formula:C11H19N
- Molecular Weight:165.28
Yield:168031-65-0 79%
Reaction Conditions:
with ammonium acetate in benzene; for 1.5 h;Reflux;Leuckart type reaction;
Steps:
Typical procedure for one-pot preparation of secondary and tertiary amines 4 (Table 1, entry 10)
To a 50-mL round-bottomed flask containing ammonium formate (0.69 g, 11 mmol) was added piperonal (1.65 g, 11 mmol) followed by pyrrolidine (0.78 g, 11 mmol) and toluene (20 mL). The mixture was magnetically stirred and heated at reflux with continuous removal of the water formed (Dean-Stark trap). The mixture was then cooled to room temperature and the solvent was removed by rotary evaporation. The crude product was purified by distillation under reduced pressure and afforded the amine.
References:
O'Connor, Danielle;Lauria, Ashley;Bondi, Steven P.;Saba, Shahrokh [Tetrahedron Letters,2011,vol. 52,# 1,p. 129 - 132] Location in patent:experimental part