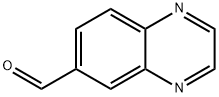
QUINOXALINE-6-CARBALDEHYDE synthesis
- Product Name:QUINOXALINE-6-CARBALDEHYDE
- CAS Number:130345-50-5
- Molecular formula:C9H6N2O
- Molecular Weight:158.16
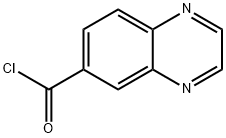
258503-93-4

130345-50-5
The general procedure for the synthesis of quinoxaline-6-carbaldehyde from quinoxaline-6-carbonyl chloride is as follows: in an 11-liter three-neck flask under argon protection, quinoxaline-6-carbonyl chloride was dissolved in 600 mL of 1,2-dimethoxyethane (DME). An equimolar amount of lithium tri-tert-butoxyaluminum hydride (LiAlH(Ot-Bu)3) was slowly added to this solution over a period of 1.5 hours at -78 °C. The reaction was stirred continuously at -78 °C for 5 hours. Upon completion of the reaction, the reaction was quenched by careful addition of ice water, followed by dilution of the reaction mixture with ethyl acetate (AcOEt) and filtration through a diatomaceous earth pad. The organic and aqueous layers were separated and the organic phase was washed with saturated sodium bicarbonate (NaHCO3) solution. After evaporation of the solvent under reduced pressure, quinoxaline-6-carbaldehyde was obtained in 73% yield and the product was a pale yellow solid. High performance liquid chromatography (HPLC) analysis showed a retention time of 1.49 min. Liquid chromatography-mass spectrometry (LC-MS) analysis showed a retention time of 0.81 min at m/z of 159.37 [M+H]+ in ESI positive ion mode. Nuclear magnetic resonance hydrogen spectroscopy (1H NMR, CDCl3) data were as follows: δ 10.28 (s, 1H), 8.97 (s, 2H), 8.61 (s, 1H), 8.27 (q, J=6 Hz, 9 Hz, 2H).
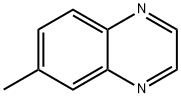
6344-72-5
144 suppliers
$8.00/100mg

130345-50-5
161 suppliers
$24.00/100mg
Yield:130345-50-5 91%
Reaction Conditions:
with selenium(IV) oxide in 1,4-dioxane at 200; for 3 h;Product distribution / selectivity;Microwave irradiation;
Steps:
1.b
b) Quinoxaline-6-carbaldehyde. A suspension of 6-methylquinoxaline (8.0 g; 0.055 mol.) and selenium dioxide (6.77 g; 0.061 mol.) in 1,4-dioxane (5.0 mL) was irradiated at 200° C. for 30 min. in a Biotage Initiator microwave synthesizer. The above procedure was repeated five further times and the combined, cooled reaction mixtures were dissolved in CH2Cl2, filtered through a plug of celite, and concentrated in vacuo. Purification via flash column chromatography (silica gel, 20-50% ethyl acetate in hexanes) followed by crystallization from CH2Cl2 provided quinoxaline-6-carbaldehyde (40.0 g, 91%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm 10.25 (s, 1H) 8.95 (s, 2H) 8.57 (d, J=1.3 Hz, 1H) 8.24 (dd, J=8.6, 1.5 Hz, 1H) 8.20 (d, J=8.6 Hz, 1H). MS(ES+) m/e 159 [M+H]+.
References:
Duffy, Kevin J.;Fitch, Duke M.;Norton, Beth A. US2007/179144, 2007, A1 Location in patent:Page/Page column 4

258503-93-4
42 suppliers
$44.00/100mg

130345-50-5
161 suppliers
$24.00/100mg
874279-33-1
4 suppliers
inquiry

130345-50-5
161 suppliers
$24.00/100mg
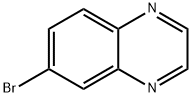
50998-17-9
198 suppliers
$6.00/250mg
201230-82-2
1 suppliers
inquiry

130345-50-5
161 suppliers
$24.00/100mg
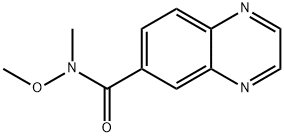
875558-38-6
21 suppliers
inquiry

130345-50-5
161 suppliers
$24.00/100mg