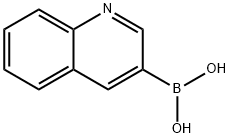
3-Quinolineboronic acid synthesis
- Product Name:3-Quinolineboronic acid
- CAS Number:191162-39-7
- Molecular formula:C9H8BNO2
- Molecular Weight:172.98
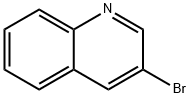
5332-24-1

191162-39-7
General procedure for the synthesis of quinoline-3-boronic acid from 3-bromoquinoline: triisopropyl borate (3.30 mL, 29.06 mmol) and 3-bromoquinoline (3.00 g, 14.49 mmol) were added to a 250 mL two-necked round-bottomed flask under nitrogen protection and dissolved in anhydrous tetrahydrofuran (100 mL). The reaction system was cooled to -78 °C and a 2M hexane solution of n-butyllithium (14.50 mL, 29.00 mmol) was slowly added dropwise through a dropping funnel over a controlled period of 1 hour. After the dropwise addition, the reaction was kept at -78 °C for 2 hours. Subsequently, the acetone/dry ice bath was removed and the reaction system was slowly warmed to 0 °C. The reaction was quenched with 2 M hydrochloric acid solution, followed by adjusting the pH with 2 M sodium bicarbonate solution to 7. The reaction mixture was extracted with ethyl acetate (3 x 100 mL). The organic phases were combined, dried over anhydrous magnesium sulfate and concentrated to dryness under reduced pressure. The product was precipitated by addition of hexane to give a white solid in 80% yield.

5332-24-1
366 suppliers
$10.00/5g

191162-39-7
307 suppliers
$10.00/1g
Yield:191162-39-7 90%
Reaction Conditions:
with n-butyllithium;Triisopropyl borate in tetrahydrofuran;hexane at -78; for 1 h;Inert atmosphere;Schlenk technique;
Steps:
2.2.5 2-Naphthalenyl boronic acid (2NB)
General procedure: To a two-neck 250mL round bottom flask, triisopropyl borate (3.30mL, 29.06mmol) and 3-bromoquinoline (3.00g, 14.49mmol) was dissolved in dry THF (100mL), then n-butyllithium (14.50mL of a 2M solution in hexane, 29.00mmol) was added dropwise via a dropping funnel over 1h under N2 at-78°C. After 2h, the acetone/dry ice bath was removed, and the reaction solution was allowed to warm to 0°C. The reaction was then quenched with a 2M HCl solution, and the pH value was adjusted to 7 with a solution of 2M NaHCO3. The resulting solution was extracted with ethyl acetate (EA) (3×100mL). The combined organic layers were dried with MgSO4 and evaporated to dryness. n-Hexane was then added to precipitated the product as a white solid (80% yield).
References:
Wang, Jianli;Leung, Louis M. [Dyes and Pigments,2013,vol. 99,# 1,p. 105 - 115]

5332-24-1
366 suppliers
$10.00/5g

5419-55-6
387 suppliers
$12.00/5g

191162-39-7
307 suppliers
$10.00/1g

5332-24-1
366 suppliers
$10.00/5g

121-43-7
381 suppliers
$13.00/25mL

191162-39-7
307 suppliers
$10.00/1g
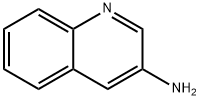
580-17-6
201 suppliers
$13.43/1gm:
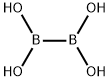
13675-18-8
167 suppliers
$6.00/1g

191162-39-7
307 suppliers
$10.00/1g

580-17-6
201 suppliers
$13.43/1gm:

191162-39-7
307 suppliers
$10.00/1g