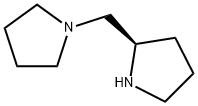
(R)-(-)-1-(2-Pyrrolidinylmethyl)pyrrolidine synthesis
- Product Name:(R)-(-)-1-(2-Pyrrolidinylmethyl)pyrrolidine
- CAS Number:60419-23-0
- Molecular formula:C9H18N2
- Molecular Weight:154.25
![Pyrrolidine, 1-[(2R)-2-pyrrolidinylcarbonyl]- (9CI)](/CAS/GIF/144243-45-8.gif)
144243-45-8

60419-23-0
Using (+)-Cbz-D-proline (1.5 g, 6 mmol) as raw material, EDC (2.3 g, 12 mmol), HOBt (800 mg, 6 mmol), TEA (1.5 mL), and pyrrolidine (853 mg, 12 mmol) were sequentially added in DMF (20 mL) and the reaction was stirred at room temperature for 18 hours. After completion of the reaction, the reaction solution was diluted with water and sodium bicarbonate and extracted with dichloromethane (3 x 20 mL). The organic phases were combined, concentrated and purified by silica gel column chromatography to afford benzyl (R)-2-(pyrrolidine-1-carbonyl)-pyrrolidine-1-carboxylate. The resulting benzyl ester of (R)-2-(pyrrolidine-1-carbonyl)-pyrrolidine-1-carboxylate was dissolved in methanol, Pd/C catalyst was added and hydrogenated at room temperature for 20 h. After completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated to give pyrrolidin-1-yl-(R)-pyrrolidin-2-yl- methanone. Pyrrolidin-1-yl-(R)-pyrrolidin-2-yl-methanone (1.2 g, 7.1 mmol) was dissolved in THF (10 mL) at 0 °C and B2H6 (10 mL, 10 mmol) was added slowly. The reaction mixture was heated to reflux and reacted for 16 hours. Upon completion of the reaction, the reaction solution was acidified with HCl and concentrated to remove the solvent. The residue was adjusted to pH 10 with 2N NaOH and extracted with dichloromethane solution containing 5% methanol. The organic phases were combined, concentrated and purified by silica gel column chromatography to afford the target product (R)-1-(2-pyrrolidinylmethyl)pyrrolidine 800 mg in 73% yield.
![Pyrrolidine, 1-[(2R)-2-pyrrolidinylcarbonyl]- (9CI)](/CAS/GIF/144243-45-8.gif)
144243-45-8
5 suppliers
inquiry

60419-23-0
102 suppliers
$45.00/10mg
Yield:60419-23-0 800 mg (73%)
Reaction Conditions:
with BH3 in tetrahydrofuran;4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran
Steps:
201 Synthesis of 5-(2,6-Dichloro-phenylmethanesulfonyl)-3-[1-[3,5-dimethyl-4-((R)-2-pyrrolidin-1-ylmethyl-pyrrolidine-1-carbonyl)-1H-pyrrol-2-yl]-meth-(Z)-ylidene]-1,3-dihydro-indol-2-one
To a solution of pyrrolidin-1-yl-(R)-pyrrolidin-2-yl-methanone (1.2 g, 7.1 mmol) in THF (10 mL) at 0° C. was added BH3 (10 mL, 10 mmol). The mixture was heated to reflux for 16 hours. The reaction was acidified with HCl and concentrated. The residue was basified to pH 10 with 2N NaOH and extracted with 5% methanol in DCM. The organic layer was concentrated and purified on a silica gel column to give 800 mg (73%) of (R)-2-pyrrolidin-1-ylmethyl-pyrrolidine.
References:
SUGEN, INC. US2003/125370, 2003, A1
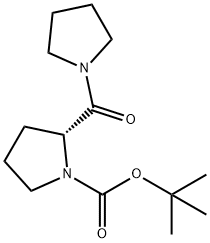
144243-44-7
1 suppliers
inquiry

60419-23-0
102 suppliers
$45.00/10mg
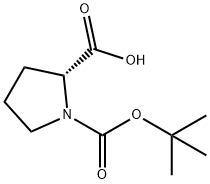
37784-17-1
426 suppliers
$6.00/5g

60419-23-0
102 suppliers
$45.00/10mg

144243-44-7
1 suppliers
inquiry

60419-23-0
102 suppliers
$45.00/10mg
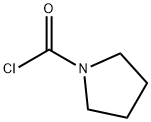
1192-63-8
90 suppliers
$26.69/1g

60419-23-0
102 suppliers
$45.00/10mg