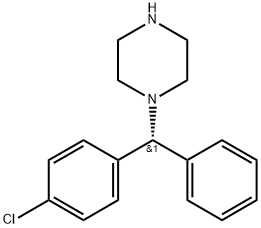
(R)-1-[(4-Chlorophenyl)phenylmethyl]piperazine synthesis
- Product Name:(R)-1-[(4-Chlorophenyl)phenylmethyl]piperazine
- CAS Number:300543-56-0
- Molecular formula:C17H19ClN2
- Molecular Weight:286.8
![chlorophenyl)phenylMethyl]-1-piperazi-necarboxylic acid, phenyl ester](/CAS/20200515/GIF/1136010-90-6.gif)
1136010-90-6
5 suppliers
$771.00/1g
![(R)-1-[(4-Chlorophenyl)phenylmethyl]piperazine](/CAS/GIF/300543-56-0.gif)
300543-56-0
199 suppliers
$11.00/250mg
Yield:~ 99 % ee
Reaction Conditions:
with sodium hydroxide;water in isopropyl alcohol; for 3 - 3.5 h;Product distribution / selectivity;Heating / reflux;
Steps:
7.2; 8.2
Above crude material was dissolved in a solution containing 4 g NaOH in 10 ml H2O and 40 ml 2-propanol. With stirring, mixture was refluxed for 3 hours.The mixture was concentrated in vacuo to get rid of 2-propanol and redissolved in 50 ml ethyl acetate and 20 ml H2O. The mixture was stirred for 20 min, and layers were separated. 20 ml HCl (2M) was added to the ethyl acetate layer and stirred for 20 min. Separated ethyl acetate layer was extracted again with 10 ml HCl (1M). Acidic layers were combined and basified. The mixture was extracted with ethyl acetate (2×50 ml). Combined ethyl acetate layer was washed with H2O (10 ml), brine (10 ml), dried and concentrated in vacuo to give the desired product (2.7 g), which was solidified while seeded. Optical purity 99%. Step 2Above crude material was mixed with 100 ml 2-propanol, 25 ml water and 13.5 g sodium hydroxide. The mixture was refluxed for 3.5 hours. It was concentrated in vacuo to get rid of 2-propanol and re-dissolved in a mixture of 100 ml toluene and 40 ml water. The mixture was stirred for 45 min. The solid was filtered off. The organic layer was separated and washed with 10 ml water, and extracted twice with 50 ml HCl (2M). After washing the aqueous layer with 25 ml toluene, the mixture was basified to pH 8-9, extracted with 100 ml toluene. The organic layer was washed with 10 ml water, dried and concentrated in vacuo to give the desired product (8 g, 60% yield). Stirring the crude product in 20 ml of toluene for 20 minutes, followed by filtrating and drying at 40° C. (vacuum oven) overnight, gave 4.5 g of the purified product.
References:
US2009/143582,2009,A1 Location in patent:Page/Page column 6
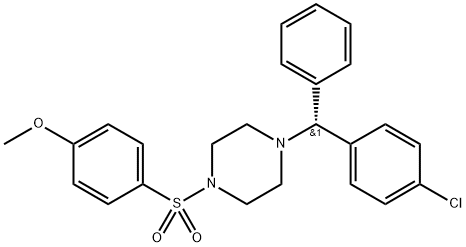
943987-59-5
1 suppliers
inquiry
![(R)-1-[(4-Chlorophenyl)phenylmethyl]piperazine](/CAS/GIF/300543-56-0.gif)
300543-56-0
199 suppliers
$11.00/250mg
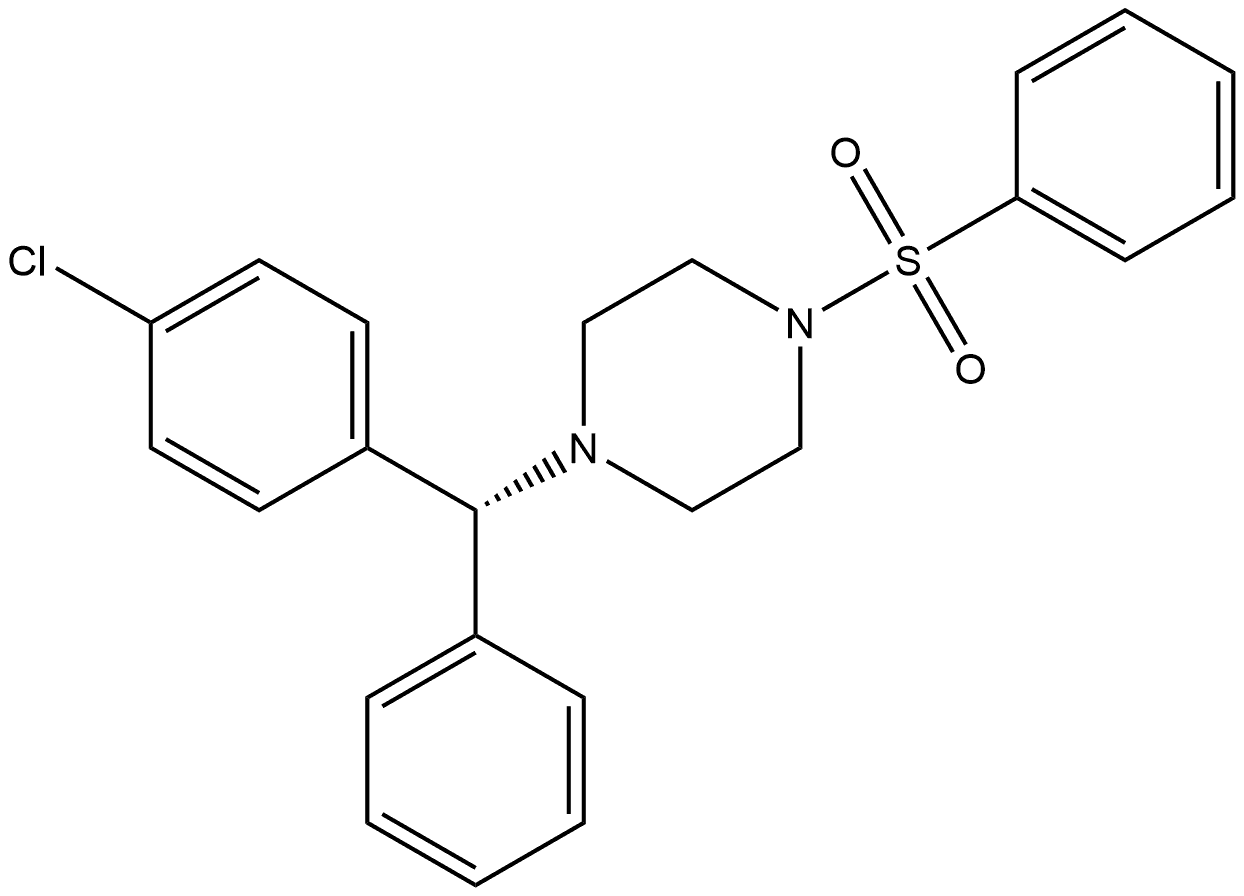
1092460-01-9
6 suppliers
inquiry
![(R)-1-[(4-Chlorophenyl)phenylmethyl]piperazine](/CAS/GIF/300543-56-0.gif)
300543-56-0
199 suppliers
$11.00/250mg
![1-Piperazinecarboxylic acid, 4-[(R)-(4-chlorophenyl)phenylmethyl]-, 2-methylpropyl ester](/CAS/20210305/GIF/1391851-21-0.gif)
1391851-21-0
0 suppliers
inquiry
![(R)-1-[(4-Chlorophenyl)phenylmethyl]piperazine](/CAS/GIF/300543-56-0.gif)
300543-56-0
199 suppliers
$11.00/250mg
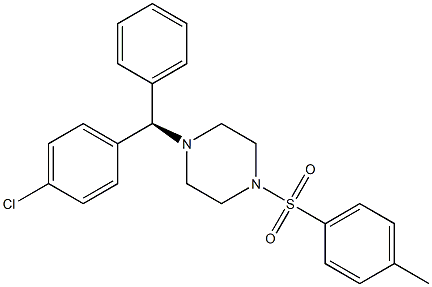
942283-97-8
5 suppliers
inquiry
![(R)-1-[(4-Chlorophenyl)phenylmethyl]piperazine](/CAS/GIF/300543-56-0.gif)
300543-56-0
199 suppliers
$11.00/250mg