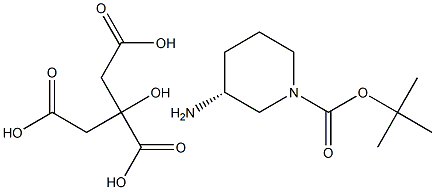
R-1-BOC-3-aMino piperidine citric acid salt synthesis
- Product Name:R-1-BOC-3-aMino piperidine citric acid salt
- CAS Number:1253790-41-8
- Molecular formula:C16H28N2O9
- Molecular Weight:392.4015
Yield:1271811-51-8 45%
Reaction Conditions:
Stage #1: (R)-tert-butyl 3-aminopiperidine-1-carboxylate, citric acid saltwith sodium hydroxide in dichloromethane; for 0.166667 h;
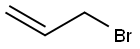
Stage #2: allyl bromidewith potassium carbonate in acetonitrile at 20;Cooling with ice;
Steps:
39
To a mixture of (R)-tert- butyl 3-aminopiperidine-l-carboxylate. critic acid 39.1 (20 g, 51 mmol) in DCM (50 mL) was added NaOH (5M, 50 mL), the mixture was stirred for 10 min and then extracted with DCM (50 mL X 3), the combined organics were washed with brine (30 mL), dried over Na2S04 and concentrated to give a colorless oil. The oil was dissolved in CH3CN (60 mL) and K2C03 (4.2 g, 30.6 mmol, 0.6 eq) was added under ice bath, then allyl bromide (2.9 mL, 34.2 mmol, 0.67 eq) in CH3CN (15 mL) was added dropwise. After the addition was finished, the mixture was warmed to rt and stirred for another 12 h. Water (10 mL) was added and the mixture was extracted with EtOAc (15 mL x 3), the combined organics were dried over Na2SC>4, concentrated in vacuo and purified by column chromatography (silica gel, DCM:MeOH = 30: 1) to afford 39.2 as a light yellow oil (5.5 g, yield: 45%). LCMS: (M+H) +: 241.1
References:
WO2011/29046,2011,A1 Location in patent:Page/Page column 212; 213