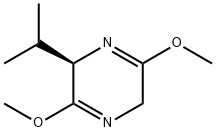
(R)-2,5-Dihydro-3,6-dimethoxy-2-isopropylpyrazine synthesis
- Product Name:(R)-2,5-Dihydro-3,6-dimethoxy-2-isopropylpyrazine
- CAS Number:109838-85-9
- Molecular formula:C9H16N2O2
- Molecular Weight:184.24
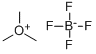
420-37-1
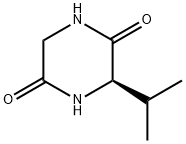
143673-66-9

109838-85-9
Step A: Preparation of (R)-2-isopropyl-3,6-dimethoxy-2,5-dihydropyrazine: To a 2L round bottom flask was added (R)-3-isopropylpiperazine-2,5-dione (20.7 g, 133 mmol), trimethine oxonium tetrafluoroborate (49.0 g, 331 mmol), and dichloromethane (500 mL). The reaction mixture was stirred vigorously at room temperature and protected by nitrogen.After 18 h, the reaction mixture changed to a clarified solution and formed a very viscous yellow oil at the bottom of the flask. Trimethyloxonium tetrafluoroborate (19.6 g, 133 mmol) was added additionally and stirring was continued at room temperature.After 23 h, the reaction mixture was cooled in an ice bath and 200 g of ice and 100 mL of concentrated ammonium hydroxide solution (28%) were added slowly. Stirring was continued in the ice bath for 1 hr. The organic and aqueous layers were separated and the aqueous layer was extracted with dichloromethane (2 x 50 mL). The organic layers were combined, washed sequentially with saturated sodium bicarbonate solution (2 x 100 mL) and brine (100 mL), dried with anhydrous potassium carbonate, filtered through a pad of diatomaceous earth, and concentrated under reduced pressure to give 25.9 g of light brown oil. The crude product was purified by column chromatography (eluent: ether/pentane=1:4) to afford 17.464 g of the target compound (R)-2-isopropyl-3,6-dimethoxy-2,5-dihydropyrazine as a colorless oil in 71.5% yield.1H NMR (400 MHz, CDCl3) δ 4.08-3.94 (m, 3H), 2.95 (s, 3H) , 2.87 (s, 3H), 2.30-2.18 (m, 1H), 1.04 (d, J=7.03Hz, 3H), 0.76 (d, J=6.64Hz, 3H).
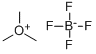
420-37-1
250 suppliers
$17.00/5g

143673-66-9
127 suppliers
$9.00/250mg

109838-85-9
196 suppliers
$40.00/500 mg
Yield:109838-85-9 71.5%
Reaction Conditions:
Stage #1:trimethoxonium tetrafluoroborate;(R)-3-(1-methylethyl)-piperazine-2,5-one in dichloromethane at 20; for 41 h;
Stage #2: with ammonia;water in dichloromethane at 0; for 1 h;
Steps:
1.A; 2.A (S)-Methyl 2-(5-(2,4-difluorophenoxy)-1-isobutyl-1H-indazole-6-carboxamido)-4-(dimethylamino)-2-methylbutanoate (9a)
Step A: Preparation of (R)-2-isopropyl-3,6-dimethoxy-2,5-dihydropyrazine (1): To a 2 L round-bottomed flask were added (R)-3-isopropylpiperazine-2,5-dione (20.7 g, 133 mmol), Me3OBF4 (49.0 g, 331 mmol) and CH2Cl2 (500 mL). The slurry was stirred vigorously at room temperature under nitrogen atmosphere. After stirring 18 hours, the slurry became a clear solution with very viscous yellow oil settling on the bottom of the flask. An additional equivalent of Me3OBF4 (19.6 g, 133 mmol) was added and the mixture was stirred at room temperature. After 23 hours, the mixture was cooled in an ice bath, and 200 g of ice and 100 mL of concentrated ammonium hydroxide solution (28%) were added to the reaction mixture. The reaction mixture was stirred in an ice bath for 1 hour. The layers were separated and aqueous layer was extracted with CH2Cl2 (2*50 mL). The combined organic layers were washed with saturated NaHCO3 solution (2*100 mL) and brine (100 mL), dried over K2CO3, filtered through a Celite pad, and concentrated under reduced pressure to provide 25.9 g of light brown oil. The crude material was purified by chromatography with 1:4 ether/pentane to provide 17.464 g of compound 1 as a colorless oil (71.5% yield). 1H NMR (400 MHz, CDCl3) δ 4.08-3.94 (m, 3H), 2.95 (s, 3H), 2.87 (s, 3H), 2.30-2.18 (m, 1H), 1.04 (d, J=7.03 Hz, 3H), 0.76 (d, J=6.64 Hz, 3H).
References:
ARRAY BIOPHARMA INC. US2006/264431, 2006, A1 Location in patent:Page/Page column 20-21
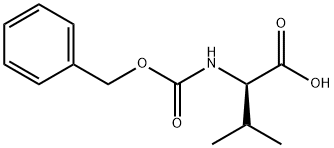
1685-33-2
261 suppliers
$8.00/1g

109838-85-9
196 suppliers
$40.00/500 mg
![Glycine, N-[(phenylmethoxy)carbonyl]-D-valyl-, methyl ester](/CAS/20210305/GIF/32402-31-6.gif)
32402-31-6
0 suppliers
inquiry

109838-85-9
196 suppliers
$40.00/500 mg
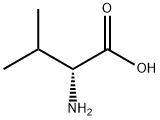
640-68-6
477 suppliers
$8.00/10g

109838-85-9
196 suppliers
$40.00/500 mg
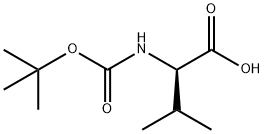
22838-58-0
301 suppliers
$5.00/1g

109838-85-9
196 suppliers
$40.00/500 mg