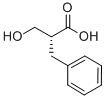
(R)-2-BENZYL-3-HYDROXYPROPANOIC ACID synthesis
- Product Name:(R)-2-BENZYL-3-HYDROXYPROPANOIC ACID
- CAS Number:123802-80-2
- Molecular formula:C10H12O3
- Molecular Weight:180.2

6811-98-9
14 suppliers
inquiry

123802-80-2
27 suppliers
inquiry
Yield: 46.5%
Reaction Conditions:
with (1S,2R)-1-amino-2-indanol in methanolReflux;
Steps:
1.1.1; 1.1.2; 1.1.3; 1.1.4; 1.1.5 1. Preparation of Intermediate I
1.1) 36.4 g (0.20 mol) of 2-benzyl-3-hydroxypropionic acid was added to 108 ml of methanol, and the solution was warmed to dissolve, and 31.33 g (0.21 mol) was added.(1S,2R)-(-)-1-amino-2-nonanol,Then warm up to reflux and carry out the incubation reaction for 30-60 minutes;1.2) After the incubation reaction, the reaction solution of step 1.1) is cooled and crystallized and filtered.The filter cake was rinsed with ice-cold methanol and dried to give 29.81 g (0.09 mol) of (1S,2R)-(-)-1-amino-2-nonanol andSalt formation of (R)-2-benzyl-3-hydroxypropionic acidThe yield was 45.0% and the bottom solution was collected;1.3) Step 1.2) the salt is added to 90 ml of dichloromethane for ice water bath;1.4) Slowly add 5% dilute hydrochloric acid to the reaction solution of step 1.3) until pH99.0%, (1S, 2R)-(-)-1- Amino-2-furfuryl alcohol 97.0%. (1S, 2R)-(-) 1-Amino-2-indolol0.30%. The methanol base collected in step 1.2) is racemized using the following method:Collect the bottom methanol solution in step 1.2), distill it under reduced pressure to dryness, add 90ml of dichloromethane, then slowly add 5% dilute hydrochloric acid till pH<1, stand still, and wash once with drinking water; add 5 %NaOH solution 50ml, reflux 8-10 smallAt the time, cooling, standing stratification, acidification with hydrochloric acid, so that pH <1, standing stratified, washed once, distilled to dry, add 54 ml of methanol, drinking water 54ml, heated to reflux, heat for 10 minutes, cooling crystallization,17.0 g (0.093 mol) of2-Benzyl-3-hydroxypropionic acid, yield 46.5%.Step 1.1) is performed again using the obtained 2-benzyl-3-hydroxypropionic acid to increase the yield of the intermediate I(R)-2-benzyl-3-hydroxypropionic acid.
References:
Shanxi Han River Pharmaceutical Group Co., Ltd.;Chen Zhi;Zhang Jia;Ding Xianshuai;Li Xuehui CN107674010, 2018, A Location in patent:Paragraph 0064; 0066; 0067; 0068; 0069; 0070-0074

110270-52-5
6 suppliers
inquiry

123802-80-2
27 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

123802-80-2
27 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

123802-80-2
27 suppliers
inquiry
![Benzenepropanoic acid, α-[(phenylmethoxy)methyl]-, (αR)-](/CAS/20210305/GIF/123803-51-0.gif)
123803-51-0
0 suppliers
inquiry

123802-80-2
27 suppliers
inquiry