
DIETHYL 2-(2-NITROBENZYL)MALONATE synthesis
- Product Name:DIETHYL 2-(2-NITROBENZYL)MALONATE
- CAS Number:59803-35-9
- Molecular formula:C14H17NO6
- Molecular Weight:295.29
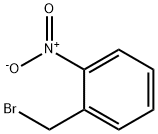
3958-60-9
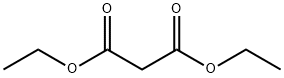
105-53-3

59803-35-9
General procedure for the synthesis of diethyl 2-(2-nitrobenzyl)malonate from 2-nitrobenzyl bromide and diethyl malonate: Potassium carbonate (9.6 g, 0.069 mol) was added to a stirring solution of diethyl malonate (8.89 g, 0.055 mol) in dimethylformamide (50 mL) under an inert atmosphere with continuous stirring for 15 minutes. Subsequently, a solution of 1-(bromomethyl)-2-nitrobenzene (10 g, 0.046 mol) in dimethylformamide (10 mL) was slowly added to the reaction mixture. The reaction mixture was stirred at room temperature for 2 h. The progress of the reaction was monitored by thin layer chromatography (TLC) to confirm complete consumption of raw materials. Upon completion of the reaction, the mixture was diluted with water (600 mL) and extracted with ethyl acetate (3 x 250 mL). The organic phases were combined, dried with anhydrous magnesium sulfate and subsequently concentrated under reduced pressure. The crude product was purified by column chromatography (silica gel: 100-200 mesh, eluent: 5% hexane solution of ethyl acetate) to give the final target compound diethyl 2-(2-nitrobenzyl)malonate (8.7 g, 63% yield).
Yield:59803-35-9 63%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 2 h;Inert atmosphere;
Steps:
7.7.1.a
To a stirred solution of diethylmalonate (8.89 g, 0.055 mol) in dimethylformamide (50 ml_), potassium carbonate (9.6 g, 0.069 mol) was added and it was stirred for 15 minutes in an inert atmosphere. 1-(bromomethyl)-2-nitrobenzene (10 g, 0.046 mol) in dimethylformamide (10 ml_) was added to the reaction mixture. The reaction mixture was stirred at ambient temperature for 2 hours. TLC showed the total consumption of the starting material. The reaction mixture was diluted with water (600 mL) and the aqueous part was extracted with ethyl acetate (3 x 250 mL). The organic part was dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by column chromatography (silica gel: 100-200 mesh, eluent: 5% ethyl acetate in hexane) to afford the pure compound (8.7g, 63% yield).
References:
WO2010/127855,2010,A1 Location in patent:Page/Page column 145
17455-13-9
645 suppliers
$5.00/10g
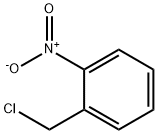
612-23-7
12 suppliers
$60.00/500mg

59803-35-9
23 suppliers
inquiry

17455-13-9
645 suppliers
$5.00/10g

612-23-7
12 suppliers
$60.00/500mg

105-53-3
793 suppliers
$5.00/25g

59803-35-9
23 suppliers
inquiry
![diethyl 2-[(2-nitrophenyl)methylidene]propanedioate](/CAS/GIF/17422-56-9.gif)
17422-56-9
20 suppliers
$60.00/100mg

59803-35-9
23 suppliers
inquiry

![Propanedioic acid, 2-[(2-nitrophenyl)methyl]-](/CAS/20210305/GIF/683215-25-0.gif)