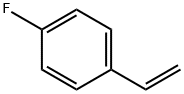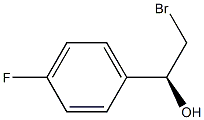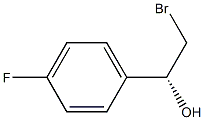
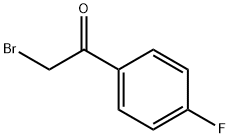
(R)-2-BROMO-1-(4-FLUOROPHENYL)ETHANOL synthesis
- Product Name:(R)-2-BROMO-1-(4-FLUOROPHENYL)ETHANOL
- CAS Number:1097211-38-5
- Molecular formula:C8H8BrFO
- Molecular Weight:219.0509
403-29-2
315 suppliers
$8.00/5g

1097211-38-5
8 suppliers
inquiry
Yield:1097211-38-5 100%
Reaction Conditions:
with dimethylsulfide borane complex;(3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole in tetrahydrofuran at 20;Inert atmosphere;stereoselective reaction;
Steps:
4.3.6. (1R)-2-bromo-1-(4-fluorophenyl)ethan-1-ol (7a) and (1S)-2-bromo-1-(4-fluorophenyl)ethan-1-ol (7b)
Reaction was carried out in dry conditions under argon atmosphere. For the synthesis of 7a, 2 M THF solution of BH3·Me2S (1.15 mL, 2.30 mmol) was added to a solution of (R)-(+)-2-methyl-CBS-oxazaborolidine catalyst (64 mg, 0.23 mmol) in anhydrous THF (2 mL). The mixture was stirred for 10 min and a solution of 2-bromo-1-(4-fluorophenyl)ethan-1-one 3 (500 mg, 2.30 mmol) in anhydrous THF (4 mL) was added dropwise. Reaction proceeded at room temperature overnight. Reaction mixture was quenched with methanol, solvent evaporated to dryness and distilled water (15 mL) added. The product was extracted with CH2Cl2 (3 × 30 mL), collected organic layers dried over Na2SO4 and solvent evaporated to dryness. 7a was purified by a silica gel column chromatography (hexane/ethyl acetate 6:1) and obtained as colourless oil (503 mg, 100%). The ee>99% of 7a was determined by chiral HPLC (200/4 Nucleodex beta-PM Column, Macherey-Nagel, Germany, Method: 0.1% TEAA in H2O: methanol 55: 45, 30 min, flow 0.7 mL/min, λ = 254 nm, retention time 19.5 min). [α]D20 = -28 (c = 1, EtOAc). 7b was synthesized following the same procedure using (S)-(-)-2-methyl-CBS-oxazaborolidine catalyst. The ee>99% was determined by chiral HPLC (retention time 18.0 min).
References:
Drai, Tonko;Molanov, Kreimir;Sachdev, Vinay;Malnar, Martina;Heimovi, Silva;Patankar, Jay V.;Obrowsky, Sascha;Levak-Frank, Sanja;Habu, Ivan;Kratky, Dagmar [European Journal of Medicinal Chemistry,2014,vol. 87,p. 722 - 734]
95895-33-3
4 suppliers
inquiry

1097211-38-5
8 suppliers
inquiry
332-42-3
154 suppliers
$15.00/1g

1097211-38-5
8 suppliers
inquiry
7589-27-7
192 suppliers
$10.00/1g

1097211-38-5
8 suppliers
inquiry