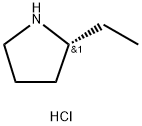
(R)-2-ethylpyrrolidine hydrochloride synthesis
- Product Name:(R)-2-ethylpyrrolidine hydrochloride
- CAS Number:460748-80-5
- Molecular formula:C6H14ClN
- Molecular Weight:135.64

1227910-54-4

460748-80-5
In a sealable reaction tube, phenylmethyl (2R)-2-ethyl-1-pyrrolidinecarboxylate (230 mg, 0.99 mmol) was dissolved in a solvent mixture of 1,4-dioxane (6 mL) and concentrated hydrochloric acid (4 mL). After sealing the reaction tube, the reaction mixture was stirred at 90°C for 5 hours. Upon completion of the reaction, the solution was concentrated and the residue was azeotropized by toluene (~20 mL) to remove residual water. Subsequently, ether (Et2O) was added to the residue and the mixture was sonicated and decanted. The solid obtained was dissolved in acetonitrile (CH3CN) and ether was added dropwise until a precipitate was generated. The mixture was again sonicated and decanted, and the solid was ground with ether to give (R)-2-ethylpyrrolidine hydrochloride (80 mg, 60% yield) as an off-white solid. The product was characterized by 1H NMR (400 MHz, CD3OD): δ 1.07 (t, J = 7.5 Hz, 3H), 1.60-1.84 (m, 3H), 1.96-2.17 (m, 2H), 2.19-2.31 (m, 1H), 3.30 (m, 2H), 3.38-3.52 (m, 1H).
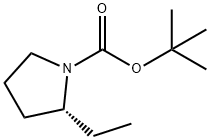
876617-06-0
33 suppliers
$26.00/250mg

460748-80-5
42 suppliers
$235.40/250mg
Yield:-
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20; for 72 h;
Steps:
Add HC1 (23 mL, 4N in 1,4-dioxane, 92 mmol) to a stirred solution of 2R-ethyl-pyrrolidine-1-carboxylic acid tert-butyl ester (2.4 g, 12 mmol) and stir at room temperature for 3 days. Concentrate under reduced pressure to afford the title compound as a colorless solid (1.6 g). *H NMR (CDCI3) 8 9.81 (br s, 1H), 9.18 (br s, 1H), 3.25-3.53 (m, 3H), 1.90-2.21 (m, 6H), 1.03-1.10 (m, 3H).
References:
WO2006/19833,2006,A1 Location in patent:Page/Page column 52
69610-40-8
398 suppliers
$6.00/5g

460748-80-5
42 suppliers
$235.40/250mg
86661-32-7
35 suppliers
$76.00/1g

460748-80-5
42 suppliers
$235.40/250mg