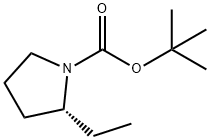
(R)-tert-butyl 2-ethylpyrrolidine-1-carboxylate synthesis
- Product Name:(R)-tert-butyl 2-ethylpyrrolidine-1-carboxylate
- CAS Number:876617-06-0
- Molecular formula:C11H21NO2
- Molecular Weight:199.29
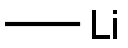
917-54-4
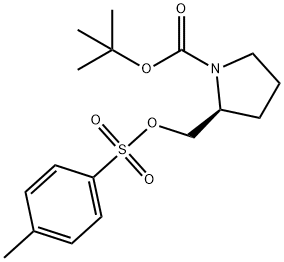
86661-32-7

876617-06-0
To a mixture of cuprous iodide (I) (9.4 g) and ether (180 ml) was added about 1 M methyl lithium/ether solution (100 ml) dropwise over a period of 30 min at an internal temperature ranging from 0 to 5°C. Stirring was continued for 15 min after completion of the dropwise addition. Subsequently, a solution of tert-butyl (2S)-2-({[(4-methylphenyl)sulfonyl]oxy}methyl)pyrrolidine-1-carboxylate (7.0 g) in dichloromethane (30 ml) was added to the reaction mixture, keeping the internal temperature at no more than 5°C, and added dropwise over 20 min. After completion of the dropwise addition, the reaction mixture was stirred at room temperature for 2.5 hours. After completion of the reaction, saturated aqueous ammonium chloride solution was added dropwise to the reaction mixture and extracted with ethyl acetate. The organic layer was dried with anhydrous sodium sulfate, filtered to remove insoluble matter and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: cyclohexane-ethyl acetate) to give tert-butyl (2R)-2-ethylpyrrolidine-1-carboxylate as an oil (3.52 g).
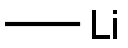
917-54-4
184 suppliers
$52.10/25ml

86661-32-7
35 suppliers
$76.00/1g

876617-06-0
33 suppliers
$26.00/250mg
Yield:876617-06-0 3.52 g
Reaction Conditions:
with copper(l) iodide in diethyl ether;dichloromethane at 0 - 20; for 3.5 h;
Steps:
47
To a mixture of copper(I) iodide (9.4g) and diethyl ether (180 ml) was added dropwise about 1M methyl lithium/diethyl ether solution (100 ml) at an internal temperature 0 to 5 °C over 30 minutes, followed by stirring 15 minutes after the dropwise addtion. to the reaction mixture tert-butyl (2S)-2-({[(4-methylphenyl)sulfonyl]oxy}methyl)pyrrolidine-1-carboxylate (7.0g) dichloromethane (30 ml) and solution was kept at an internal temperatureof 5 °C or lower and added dropwise over 20 minutes, and stirred for 2.5 hours at room temperature. A reaction mixture of a saturated ammonium chloride aqueous solution dropping a and, extracted to ethyl acetic acid. Organic layer after dry sodium sulfate anhydride, insoluble filtration fractionation and, the concentrated or depressurization of the fluid bag for filtrate. Residue silica gel column chromatography (cyclohexan-acetic acid ethyl) for purifying the, tert-butyl (2R)-2-ethylpyrrolidine-1-carboxylate (3.52g) obtained as an oily substance.
References:
KR2015/120516,2015,A Location in patent:Paragraph 0412; 0413
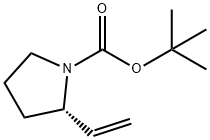
115393-77-6
25 suppliers
inquiry

876617-06-0
33 suppliers
$26.00/250mg
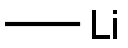
917-54-4
184 suppliers
$52.10/25ml
![tert-butyl(2S)-2-{[(methylsulfonyl)oxy]methyl}-pyrrolidine-1-carboxylate](/CAS/20180529/GIF/132482-09-8.gif)
132482-09-8
12 suppliers
inquiry

876617-06-0
33 suppliers
$26.00/250mg

86661-32-7
35 suppliers
$76.00/1g

876617-06-0
33 suppliers
$26.00/250mg
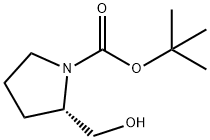
69610-40-8
398 suppliers
$6.00/5g

876617-06-0
33 suppliers
$26.00/250mg