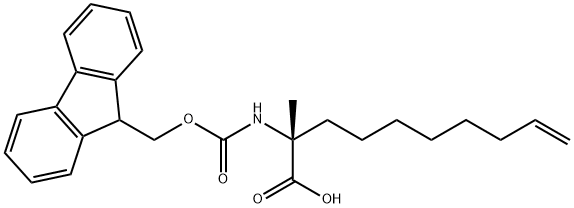
(R)-N-Fmoc-2-(7'-octenyl) alanine synthesis
- Product Name:(R)-N-Fmoc-2-(7'-octenyl) alanine
- CAS Number:945212-26-0
- Molecular formula:C26H31NO4
- Molecular Weight:421.53
![9-Decenoic acid, 2-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-2-methyl-, (2R)-, compd. with cyclohexanamine (1:1)](/CAS/20211123/GIF/1609117-04-5.gif)
1609117-04-5

945212-26-0
1. 1.55 kg (1.0 eq.) of 37-amino-2-methyl-dec-9-enoic acid hydrochloride (XIII) was suspended in 22 L of water and polished and filtered to remove traces of D-BPB hydrochloride. 2. Methyl tert-butyl ether was added and the aqueous product layer was extracted once with methyl tert-butyl ether. 3. The aqueous product layer was added again and 7 L of tetrahydrofuran was added. 4. 20% aqueous sodium carbonate (2.75 eq.) was added to the mixture followed by Fmoc-OSu (0.89 eq.). 5. The reaction was carried out at 20-25 °C, maintaining the pH at 8.5-9.0 by adding additional amounts of 20% sodium carbonate solution until the reaction was complete. 6. Adjust the pH of the mixture to 2.0-2.5 with concentrated hydrochloric acid. 7. The tetrahydrofuran is removed by distillation and methyl tert-butyl ether is added. 8. Separate the layers and wash the organic layer with water 3 times. 9. The organic layer is concentrated in vacuum and azeotropically distilled with methyl tert-butyl ether. 10. The crude oily substance is dissolved in methyl tert-butyl ether, cyclohexylamine (1.10 eq.) is slowly added, and the pH is adjusted to 8.5-9.0. 11. The slurry was stirred at 20-25 °C for 3 h. The solid product salt (XIV) was isolated by filtration. 12. The solids were rinsed twice with methyl tert-butyl ether and the wet cake was reloaded into a clean reactor. 13. The wet cake was recrystallized with tetrahydrofuran and methyl tert-butyl ether to improve purity. 14. The solid salt is suspended in methyl tert-butyl ether and water and the pH is adjusted to 2.0-2.5 with 25% sulfuric acid. 15. The organic product layer was washed with water until no cyclohexylamine remained. 16. The organic layer was concentrated and azeotropically distilled with hexane to a loose oil. 17. 17. The product (IIa) was crystallized from chloroform and hexane and purged dry with 1.0 cfm nitrogen at <0 °C. The product (IIa) was then purged dry at <0 °C with 1.0 cfm nitrogen. Yield: 1.12 kg, 41.5% yield. Recrystallization procedure: 18. Acetonitrile (23 mL/10 g of raw material ((R)-Ala-Ni-BPB(XI) in oil)) was added to the crude product and the mixture was heated to 70 °C and held for 30 min, then cooled to 20 °C. The mixture was purified with aqueous nitrogen. 19. The mixture was filtered and the solids were washed with acetonitrile (5 mL) and methyl tert-butyl ether (8.5 mL) to give a crystalline product.
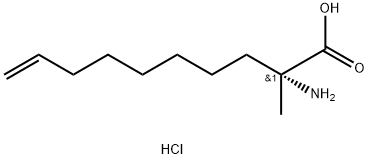
1609117-03-4
3 suppliers
inquiry
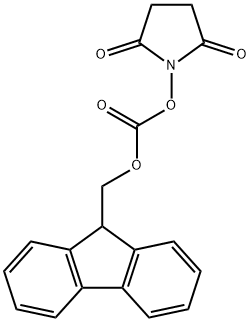
82911-69-1
669 suppliers
$7.00/25g

945212-26-0
125 suppliers
$41.00/100mg
Yield:-
Reaction Conditions:
with sodium carbonate in tetrahydrofuran;water; pH=8.5 - 9 at 20 - 25;Large scale;
Steps:
I; 1.1g Example 1g Preparation of Crystalline N-Fmoc-(R)-α-methyl-α-aminodec-9-enoic acid
Example 1g Preparation of Crystalline N-Fmoc-(R)-α-methyl-α-aminodec-9-enoic acid 1.55 kg (1.0 equiv.) of 37 2-amino-2-methyl-dec-9-enoic acid.HCl (XIII) was suspended in 22 water and polished filtered to remove trace amounts of D-BPB.HCl from the solution. 17 Methyl tert-butyl ether was added and the aqueous product layer was extracted once with methyl tert-butyl ether. The aqueous product layer was re-charged and 7 tetrahydrofuran was added. A 20% aqueous sodium carbonate solution (2.75 equiv.) was charged to the mixture followed by Fmoc-OSu (0.89 equiv.). The mixture was allowed to react at 20-25° C. while maintaining the pH between 8.5-9.0 with additional amounts of the 20% 42 sodium carbonate solution until the reaction was complete. The mixture was pH adjusted down to pH 2.0-2.5 with conc. hydrochloric acid. Tetrahydrofuran was distilled off and methyl tert-butyl ether was charged. The layers were separated and the organic layer was washed 3 more times with additional water. The organic layer was then concentrated under vacuum and co-stripped with methyl tert-butyl ether. The resulting crude 4 oil was re-dissolved in methyl tert-butyl ether and cyclohexylamine (1.10 equiv.) was added slowly to obtain a pH range of 8.5-9.0. The slurry was agitated at ambient temperature (20-25° C.) for 3 hours and the solid product salt (XIV) was isolated by filtration. The solids were rinsed twice with additional methyl tert-butyl ether and the solid wetcake was recharged to a clean reactor. The wetcake was recrystallized from tetrahydrofuran and methyl tert-butyl ether to improve the purity. The solid salt was suspended in methyl tert-butyl ether and water and the pH adjusted to 2.0-2.5 with 25% sulfuric acid. The organic product layer was washed with water until all of the cyclohexylamine was removed. The organic product layer was concentrated and co-stripped with hexanes to a loose oil. The product (IIa) was then crystallized out of chloroform and hexanes and dried at <0° C. under a 1.0 cfm nitrogen sweep. Yield: 1.12 kg, 41.5%
References:
Aileron Therapeutics, Inc.;Darlak, Krzysztof;Kawahata, Noriyuki;Athamneh, Sameer Ahmed US2014/128581, 2014, A1 Location in patent:Paragraph 0087; 0156; 0157