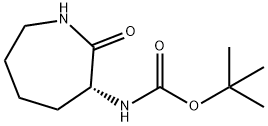
(R)-tert-butyl 2-oxoazepan-3-ylcarbaMate synthesis
- Product Name:(R)-tert-butyl 2-oxoazepan-3-ylcarbaMate
- CAS Number:106691-72-9
- Molecular formula:C11H20N2O3
- Molecular Weight:228.29
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

106691-72-9
44 suppliers
$241.66/0.25g
Yield: 95.9%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; under 7500.75 Torr;Autoclave;Inert atmosphere;Reagent/catalyst;
Steps:
4 Example 4: (R) -α-tert-butoxycarbonylamino-ε-caprolactam I
The reaction is as follows:At room temperature,To the autoclave was added 10% palladium carbon (22.6 g)Additional methanol (2.5 L) and intermediate VII (452 g, 2 mol) were added,Turn on stirringVacuum,Purged with nitrogen three times,Hydrogenation pressure to 1MPa after three times of replacement with hydrogen,TLC detection reaction,Until the point of disappearance of raw materials,filter,After the filtrate was evaporated under reduced pressure to remove the solvent,The residue is recrystallized from methanol,The resulting white solid was (R) -α-tert-butoxycarbonylamino-ε-caprolactam (437.2 g, 1.92 mol)Yield 95.9%.HPLC test product purity greater than 99%ee value greater than 99.5%.
References:
Changzhou Yabang Pharmaceutical Co., Ltd.;Chen Zaixin;Yu Shuitao;Zhou Weiyou;Zhang Mingguang;Ji Xiaolong;Lu Xufang CN106588771, 2017, A Location in patent:Paragraph 0080; 0081; 0082; 0083; 0084; 0085; 0086; 0087
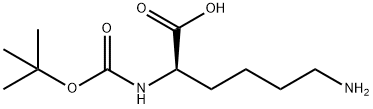
106719-44-2
163 suppliers
$11.00/1g

106691-72-9
44 suppliers
$241.66/0.25g

24424-99-5
868 suppliers
$13.50/25G
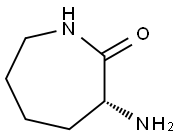
28957-33-7
142 suppliers
$45.00/50mg

106691-72-9
44 suppliers
$241.66/0.25g
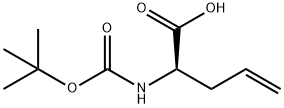
170899-08-8
148 suppliers
$24.00/1g

106691-72-9
44 suppliers
$241.66/0.25g