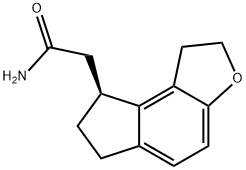
Ramelteon Impurity 6 synthesis
- Product Name:Ramelteon Impurity 6
- CAS Number:221530-38-7
- Molecular formula:C13H15NO2
- Molecular Weight:217.26
![(R)-2-(2,6,7,8-tetrahydro-1H-indeno[5,4-b]furan-8-yl)acetic acid](/CAS/GIF/1092507-02-2.gif)
1092507-02-2
14 suppliers
inquiry

221530-38-7
16 suppliers
inquiry
Yield:221530-38-7 94%
Reaction Conditions:
Stage #1: (S)-2-(2,6,7,8-tetrahydro-1H-indeno[5,4-b]furan-8-yl)acetic acidwith chloroformic acid ethyl ester;triethylamine in dichloromethane at -10 - 5;
Stage #2: with ammonia in dichloromethane at 0 - 5; for 1 h;
Steps:
6
A solution of (85)-l,2,6,7-tetrahydro-8/f-indeno[5,4-δ]furan-8-yl)acetic acid (35.0 gr, 0.1605 mol) in 350 ml of dichloromethane was cooled to between -100C. Triethylamine (19.25 gr, 0.1905 mol) was added dropwise to the reaction mixture, and the obtained reaction mixture was maintained at 00C. The reaction mixture was cooled to -100C and ethyl chloroformate (19.95 gr, 0.1838 mol) was added dropwise to the reaction with cooling, which was then stirred for 2 hours at 0-50C. Then, the reaction mixture was quenched in 550 ml of 2-5 % ammonia solution in dichloromethane at 0-50C,. The reaction mixture was stirred for an hourl at 0-50C. The solvent was distilled out under vacuum at 40 -450C and 245 ml of water and sodium bicarbonate (8.75 gr) were added. 2-[(8S)-1, 6,7, 8-tetrahydro-2/f-indeno[5,4- δ]furan-8-yl]acetamide was isolated by filtration and washed with water. Yield: 94%. Purity: 99.79%.
References:
WO2010/45565,2010,A1 Location in patent:Page/Page column 33-34
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
255 suppliers
$31.00/250mg

221530-38-7
16 suppliers
inquiry
![2H-Indeno[5,4-b]furan-8-acetyl chloride, 1,6,7,8-tetrahydro-, (8S)-](/CAS/20210305/GIF/1149757-31-2.gif)
1149757-31-2
0 suppliers
inquiry

221530-38-7
16 suppliers
inquiry
496-16-2
367 suppliers
$7.00/5g

221530-38-7
16 suppliers
inquiry
![2,3-Dihydrobenzo[b]furan-5-carbaldehyde](/CAS/GIF/55745-70-5.gif)
55745-70-5
256 suppliers
$6.00/1g

221530-38-7
16 suppliers
inquiry