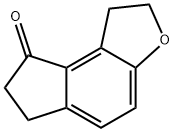
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one synthesis
- Product Name:1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one
- CAS Number:196597-78-1
- Molecular formula:C11H10O2
- Molecular Weight:174.2
![4,5-Dibromo-1,2,6,7-tertahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-77-0.gif)
196597-77-0
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
4,5-Dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (280 kg, 843 mol) was used as a raw material, mixed with anhydrous sodium acetate (173 kg, 2109 mol) and methanol (6384 L). After the reaction system was replaced with nitrogen, 10% Pd/C catalyst (30.8 kg, dry weight) was added. Subsequently, the reaction mixture was pressurized to 0.29 to 0.49 MPa under a hydrogen atmosphere and catalytically reduced for 8 hours at about 40°C with a stirring rate such that the total gas-liquid mass transfer coefficient, KLa (1/hr), was about 15. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under reduced pressure. Water was added to the concentrated residue, concentrated again under reduced pressure to displace the solvent, cooled and stirred for 1 hour to mature the crystals. The crystallized solution was filtered to give wet 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one crystals (dry weight 127 kg, yield 86.6%). The amount of dimer in the wet crystals was less than 0.1 wt%.
![4,5-Dibromo-1,2,6,7-tertahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-77-0.gif)
196597-77-0
84 suppliers
$110.00/25mg
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
256 suppliers
$31.00/250mg
Yield:196597-78-1 88.6%
Reaction Conditions:
with hydrogen;sodium acetate;palladium 10% on activated carbon in methanol at 40; under 2175.22 - 3675.37 Torr; for 8 h;
Steps:
1.1
4,5-Dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (280 kg, 843mol), anhydrous sodium acetate (173 kg, 2109 mol), methanol (6384 L) were mixed, and the reaction system was replaced with nitrogen. Then, to the reaction mixture was added 10% Pd/C (30.8 kg, as dry weight), and pressurized with hydrogen to 0.29 to 0.49 MPa, and catalytically reduced at about 40°C for 8 hrs with stirring at such a stirring rate that the gas-liquid overall mass transfer coefficient KLa(1/hr) is about 15. The catalyst was filtered, and the filtrate was concentrated under reduced pressure, and further water was added to the residue, followed by concentrating under reduced pressure to substitute the solvent, cooling and stirring for 1 hr to mature. The crystallization solution was filtered to give wet crystals of title compound (amount 127 kg as dry weight, yield 86.6%). The content of dimer in the wet crystals was less than 0.1% by weight.
References:
Takeda Pharmaceutical Company Limited EP1792899, 2007, A1 Location in patent:Page/Page column 13
![8H-Indeno[5,4-b]furan-8-one, 6,7-dihydro-](/CAS/20200611/GIF/15480-25-8.gif)
15480-25-8
1 suppliers
inquiry
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
256 suppliers
$31.00/250mg
![4,5-Dibromo-1,2,6,7-tertahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-77-0.gif)
196597-77-0
84 suppliers
$110.00/25mg
![2H-Indeno[5,4-b]furan, 1,6,7,8-tetrahydro-](/CAS/20210111/GIF/1092507-06-6.gif)
1092507-06-6
0 suppliers
inquiry
![2,6,7,8-tetrahydro-1H-indeno[5,4-b]furan-8-ol](/CAS/GIF/1092507-07-7.gif)
1092507-07-7
11 suppliers
inquiry
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
256 suppliers
$31.00/250mg
50-00-0
896 suppliers
$10.00/25g
1205098-82-3
10 suppliers
inquiry
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
256 suppliers
$31.00/250mg
496-16-2
370 suppliers
$7.00/5g
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
256 suppliers
$31.00/250mg