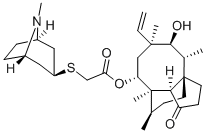
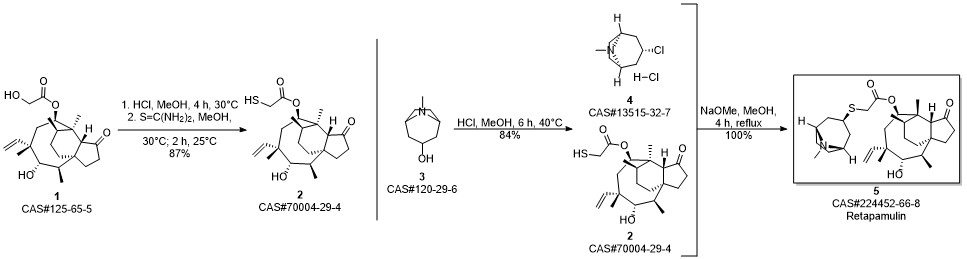
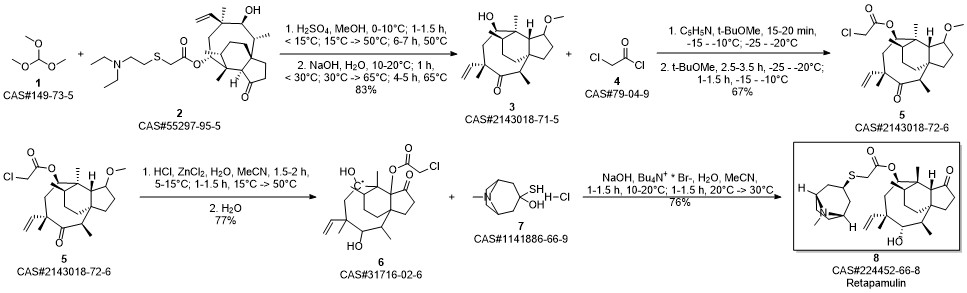
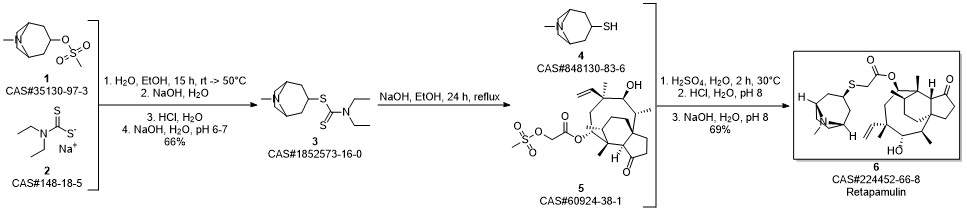
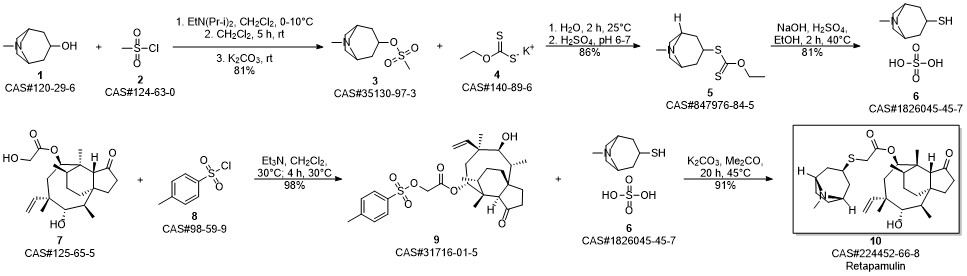
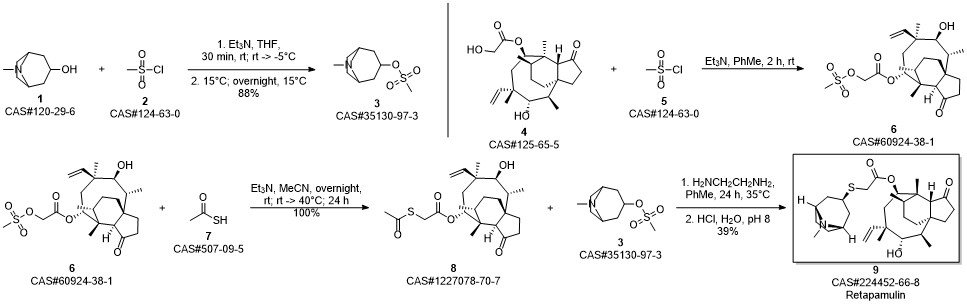
RETAPAMULIN synthesis
- Product Name:RETAPAMULIN
- CAS Number:224452-66-8
- Molecular formula:C30H47NO4S
- Molecular Weight:517.76
Breen, Gary Francis; Forth, Michael Anthony; Kopelman, Susan Shumei Hu; Muller, Francis Xavier; Sanderson, Francis Dominic. Process for preparation of mutilin derivatives and their salts as antibacterial agents. Assignee Glaxo Group Limited, UK. WO 2005023257. (2005).
Yield:224452-66-8 80%
Reaction Conditions:
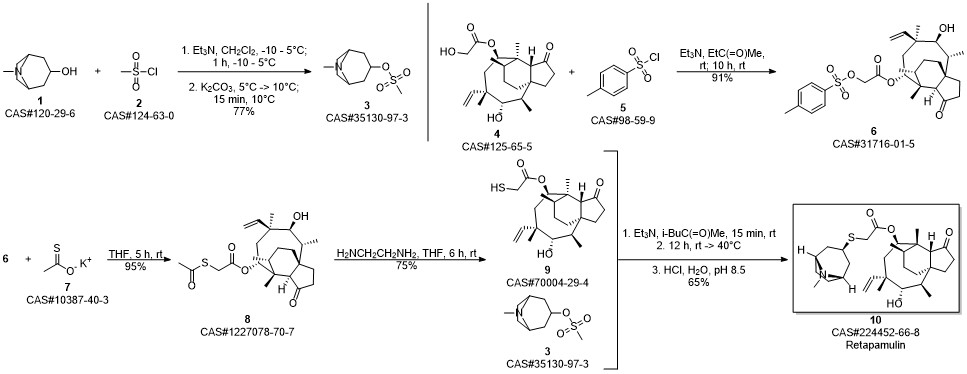
with triethylamine in 4-methyl-2-pentanone at 20 - 40; for 2 - 24 h;Product distribution / selectivity;
Steps:
17; 18 Preparation of Retapamulin
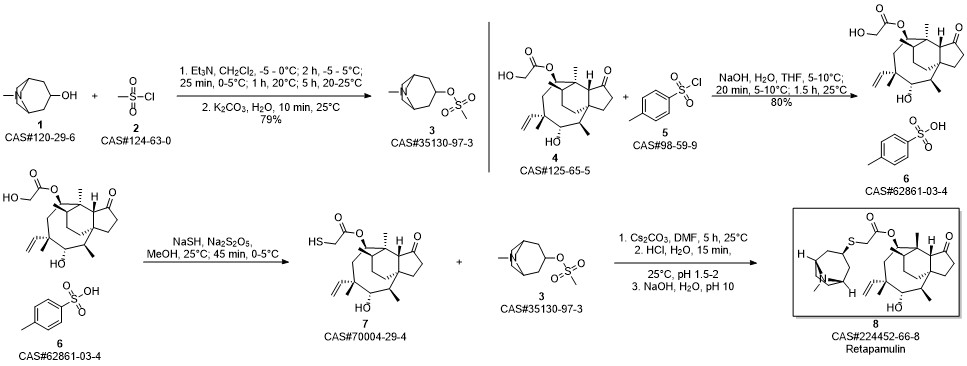
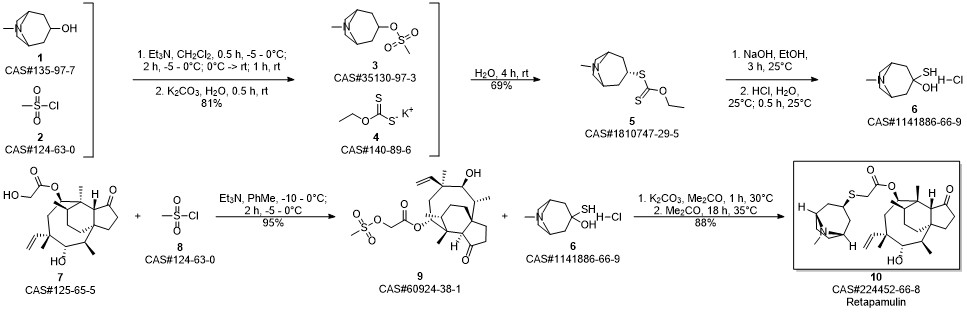
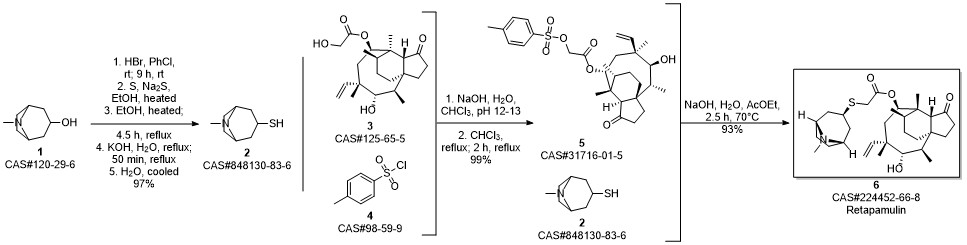
Example 17 Preparation of Retapamulin A 3 neck-flask (100 ml) was charged with tropine thiol (5-10 mmol), a solvent (5 vol) and a base (2.5 eq). The solvents and bases are listed in Table 1 below. Pleuromutilin mesylate (11 mmol) was then added to the flask in portions. The resulting combination was stirred at room temperature to 40° C. for 2-24 hours until full conversion to Retapamulin was achieved. Example 18Preparation of RetapamulinIn a 500 ml round bottom flask, tropine thiol (15 g), methyl isobutyl ketone (8 vol) and triethylamine (2.4 eq.) were charged. The mixture was stirred at 40° C. for 15 minutes. Pleuromutilin mesylate (1 eq.) was added. The mixture was stirred as a slurry at 40° C. for 12 hours. Water (7 vol) was added and the pH was adjusted to 8.5 using 4N HCl. The phases were separated. Water (7 vol) was added to the organic phase, the pH was adjusted to 8.2, and the phases separated again. The organic phase was extracted with water (7 vol) and the pH was adjusted to 1.5. After separation, the pH of the aqueous phase was adjusted to 12.5 with 4N NaOH. The mixture was stirred at room temperature for 20 hours. The product was vacuum filtered and washed with water. The collected crystals were dried in a 55° C. vacuum oven to yield 80% Retapamulin at 99.8% purity as determined by HPLC.
References:
Hedvati, Lilach;Gilboa, Eyal;Avhar-Maydan, Sharon;Shachan-Tov, Sharona US2009/149655, 2009, A1 Location in patent:Page/Page column 3; 5; 6
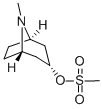
35130-97-3
41 suppliers
$151.00/250mg

224452-66-8
225 suppliers
$5.00/5mg
120-29-6
397 suppliers
$10.00/5g

224452-66-8
225 suppliers
$5.00/5mg
![8-Azabicyclo[3.2.1]octane-3-thiol, 8-Methyl-, (3-exo)-](/CAS/20180906/GIF/848130-83-6.gif)
![2-[(Methylsulfonyl)oxy]acetic acid (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten-8-yl ester](/CAS/GIF/60924-38-1.gif)