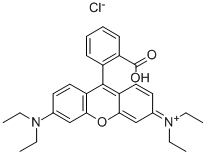
Rhodamine B synthesis
- Product Name:Rhodamine B
- CAS Number:81-88-9
- Molecular formula:C28H31ClN2O3
- Molecular Weight:479.01
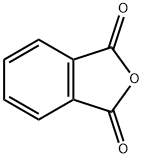
85-44-9
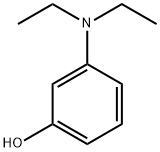
91-68-9

81-88-9
(1) Phthalic anhydride (15.7 g) and 3-(N,N-dimethylamino) phenol (18.0 g) were added to 1,2-dichlorobenzene (57.0 g). The resulting mixture was stirred at 175 °C for 1 hour and then 3-(N,N-diethylamino) phenol (12.1 g) was added in three batches. After the addition was completed, the reaction mixture was continued to be stirred at 175 °C for 12 hours. After completion of the reaction, the mixture was cooled to below 25°C. Subsequently, 3% aqueous sodium hydroxide solution (100 g) was added and stirred for 30 minutes. After separating the organic layer, 4.5% sulfuric acid (330 g) was added and stirred for 30 minutes. After separation of the aqueous layer, 35% hydrochloric acid (30 g) and sodium chloride (15 g) were added and the mixture was stirred at 80 °C for 1 hour. After cooling to room temperature, the resulting crystals were filtered, washed with 2% hydrochloric acid (300 g) and dried at 80 °C to give the compound of [chemical formula Di] (35 g).
Yield:81-88-9 35 g
Reaction Conditions:
Stage #1: phthalic anhydride;3-diethylaminophenol in 1,2-dichloro-benzene at 175; for 13 h;
Stage #2: with water;sodium hydroxide at 25; for 0.5 h;
Stage #3: with hydrogenchloride;sodium chloride in water at 80; for 1 h;
Steps:
2.1 10063] Synthesis Example 2. Synthesis of compound of [Chemical Formula 4]
10063] Synthesis Example[Chemical Formula 4]10064] (1) Afier adding phthalic anhydride (15.7 g) and3-(N,N-dimethylamino)phenol (18.0 g) to 1 ,2-dichloroben- zene (57.0 g) in a reactor, the resulting mixture was stirred at 175° C. for 1 hout 1 hour later, 3-(N,N-diethylamino)phenol (12.1 g) was added in three aliquots. Afier the addition was completed, the mixture was stirred at 175° C. for 12 hours. Upon completion of reaction, the mixture was cooled to below 25°C. and, afier adding 3% sodium hydroxide aqueous solution (100 g), stirred for 30 minutes. After separating the organic layer and adding 4.5% sulfuric acid (330 g), the mixture was stirred for 30 minutes. Afier separating the aqueous layer and adding 35% hydrochloric acid (30 g) and sodium chloride (15 g), the mixture was stirred at 80° C. for 1 hour. After cooling to room temperature, the resulting crystals were filtered, washed with 2%: hydrochloric acid (300 g) and dried at 80° C. to obtain a compound of [Chemical Formula Di (35 g).
References:
US2015/322265,2015,A1 Location in patent:Paragraph 0064
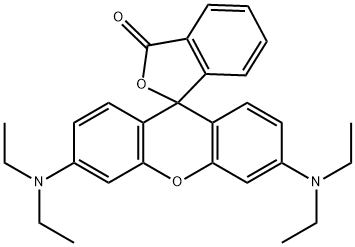
509-34-2
152 suppliers
$12.00/5g

81-88-9
506 suppliers
$24.39/50g
![Xanthylium, 3,6-bis(diethylamino)-9-[2-(hydrazinylcarbonyl)phenyl]-](/CAS/20211123/GIF/760154-10-7.gif)
760154-10-7
0 suppliers
inquiry

81-88-9
506 suppliers
$24.39/50g
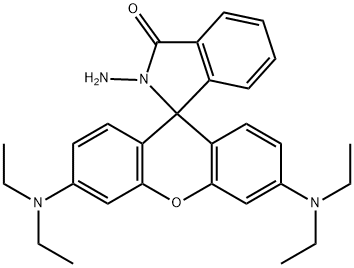
74317-53-6
59 suppliers
$28.00/5mg

81-88-9
506 suppliers
$24.39/50g

4344-42-7
4 suppliers
inquiry

81-88-9
506 suppliers
$24.39/50g