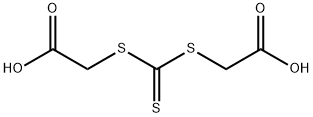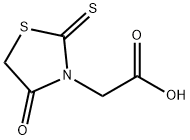
Rhodanine-3-acetic acid synthesis
- Product Name:Rhodanine-3-acetic acid
- CAS Number:5718-83-2
- Molecular formula:C5H5NO3S2
- Molecular Weight:191.23
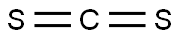
75-15-0
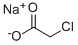
3926-62-3
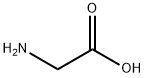
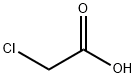
56-40-6

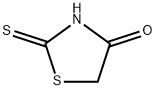
5718-83-2
GENERAL METHOD: Glycine (1 mol), 22% sodium hydroxide solution and carbon disulfide (1 mol) were dissolved in 3 mL of water and placed in a microwave reactor (CEM Discover, Benchmate, USA) and reacted at 100°C for 5 min. After the reaction was completed, it was automatically cooled to 40 °C, sodium chloroacetate (1 mol) was added and the mixture was again reacted at 100 °C for 5 min. The reaction solution was cooled to 40 °C and concentrated. To the concentrate, 3 mL of hydrochloric acid was added and the reaction was continued at 110°C for 20-30 minutes. Upon completion of the reaction, the crude product was extracted with ethyl acetate and purified (refer to Nitsche and Klein, 2012 method). The product obtained was 2-(4-oxo-2-thioxothiazolidin-3-yl)acetic acid (2a) in 82.5% yield; melting point 245-247 °C; UV λmax 266, 215 nm; IR (KBr) νmax 3439, 1663, 1512, 1321, 896 cm-1; 1H NMR (DMSO-d6, 400 MHz): δ= 4.56 (2H, s, N-CH2), 4.41 (2H, s, H-5); 13C NMR (DMSO-d6, 100 MHz): δ= 202.80 (C=S, C-2), 173.72 (C=O, COOH), 167.29 (C=O, C-4), 44.77 (CH2, N-CH2), 35.94 ( CH2, C-5); elemental analysis (C5H5NO3S2) calculated values: C, 31.40; H, 2.64; N, 7.32. measured values: C, 31.55; H, 2.53; N, 7.22.
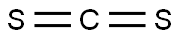
75-15-0
273 suppliers
$40.00/100 g
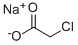
3926-62-3
300 suppliers
$16.69/250g

56-40-6
1339 suppliers
$5.00/25g

5718-83-2
339 suppliers
$5.00/1g
Yield:5718-83-2 82.5%
Reaction Conditions:
Stage #1:carbon disulfide;glycine with sodium hydroxide in water at 100; for 0.0833333 h;Microwave irradiation;
Stage #2:sodium monochloroacetic acid in water at 40 - 100; for 0.0833333 h;Microwave irradiation;
Steps:
General procedure for the microwave-assisted synthesisof N-substituted rhodanines (2a-g)
General procedure: Mixture of amine or amino acid (1 mol), sodium hydroxide (22 %) and carbon disulfide (1 mol) in 3 ml water was reacted in a microwave reactor (CEM Discover, Benchmate,USA) for 5 min at 100 °C at 80 W. After automatedcooling to 40 °C, sodium chloroacetate (1 mol) was addedand the mixture was reacted again at 100 °C for 5 min.After cooling (40 °C), conc. HCl (3 ml) was added and thereaction was completed at 110 C for 20-30 min. Thecrude product was extracted with ethyl acetate and purified(Nitsche and Klein, 2012).2-(4-Oxo-2-thioxothiazolidin-3-yl)acetic acid (2a) Yield;82.5 %; mp 245-247 °C; UV λmax 266, 215 nm; IR(KBr)νmax 3439, 1663, 1512, 1321, 896 cm-1; 1H NMR(DMSO-d6, 400 MHz): δ = 4.56 (2H, s, N-CH2), 4.41(2H, s, H-5); 13C NMR (DMSO-d6, 100 MHz): δ= 202.80(C = S, C-2), 173.72 (C = O, COOH), 167.29 (C = O,C-4), 44.77 (CH2, N-CH2), 35.94 (CH2, C-5); Anal.calcd.for C5H5NO3S2: C, 31.40; H, 2.64; N, 7.32. Found: C,31.55; H, 2.53; N, 7.22.
References:
Ali Muhammad, Sulaiman;Ravi, Subban;Thangamani, Arumugam [Medicinal Chemistry Research,2016,vol. 25,# 5,p. 994 - 1004]

29677-65-4
10 suppliers
inquiry

5718-83-2
339 suppliers
$5.00/1g