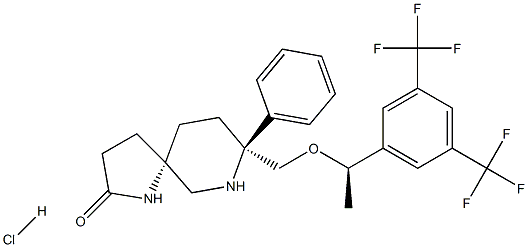
rolapitant hydrochloride (anhydrous) synthesis
- Product Name:rolapitant hydrochloride (anhydrous)
- CAS Number:858102-79-1
- Molecular formula:C25H27ClF6N2O2
- Molecular Weight:536.9375
![methyl 3-[(3S,6S)-6-({(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy}methyl)-3-nitro-6-phenylpiperidin-3-yl]propanoate methylsulfonate](/CAS/20200331/GIF/1065272-06-1.gif)
1065272-06-1
2 suppliers
inquiry

858102-79-1
12 suppliers
inquiry
Yield:858102-79-1 97 %Chromat.
Reaction Conditions:
Stage #1: methyl 3-[(3S,6S)-6-({(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy}methyl)-3-nitro-6-phenylpiperidin-3-yl]propanoate methylsulfonatewith acetic acid;zinc at 20 - 60;
Stage #2: with hydrogenchloride in ethanol;water;isopropyl alcohol;toluene at 0 - 25;
Steps:
6A
Example 6APreparation of the Compound of Formula I from 27a-SulfonateA suspension was made by adding zinc powder (12.2 Kg, 186.6 moles) to 42 liters of concentrated acetic acid with vigorous stirring. In a separate vessel was placed 4.04 Kg of the starting material prepared in Example 5, above, and 4.1 Kg of a compound 27a-sulfonate compound prepared in a similar reaction which yielded a salt comprising 88.2% S-enantiomer and 7.8% R-enantiomer (total 8.14 kg of the sulfonate salts, about 95% S-enantiomer). The sulfonate salts were dissolved in 82 liters of concentrated acetic acid heated to 45° C. to obtain a solution. When all of the solids had dissolved the solution temperature was adjusted and maintained at a temperature between 20° C. and 30° C. The solution containing the compound of Formula 27a-sulfonate was added to the stirring zinc suspension while maintaining the mixture at a temperature below 60° C. After all of the solution was added, the reaction mixture temperature was adjusted and maintained at a temperature of from 55° C. to 60° C. until the reaction was complete, as determined by HPLC. At the end of the reaction the reaction mixture was then cooled and maintained at a temperature of from 20° C. to 30° C.The reaction mixture was filtered through Hyflo (4.12 kg) and the wet cake was washed with toluene. The wash was combined with the filtrate and the mixture was concentrated under vacuum (80 mbar to 120 mbar) by maintaining the reaction mixture temperature between 30° C. and 60° C. until distillation ceased. To the concentrate was added 41 L of toluene. The resulting organic solution was washed successively with aliquots of 2N hydrochloric acid solution (45 L), sodium carbonate solution (2 aliquots of 82 L each, 8% solution) and sodium chloride solution (22 L, 10% solution). The washed solution was filtered and the filter rinsed with toluene which was combined with the filtrate. The filtrate was seeded with seed crystals of the compound of Formula I maintaining the filtrate at a temperature between 20° C. and 25° C. Concentrated hydrochloride acid was slowly added to the filtrate followed by fine spirit (95:5 ethanol/isopropanol) maintaining the mixture at a temperature between 20° C. and 25° C. The mixture was agitated at 20° C. to 25° C. for 25 to 35 minutes and then cooled to 0° C. to 5° C. and agitated for 25 to 35 minutes. The mixture was filtered and the wet cake washed with an aliquot of a 1:1 mixture of toluene/MTBE (10 L), followed by a second aliquot of MTBE (10 L) maintained at 20° C. to 25° C. The wet cake was dried at 40° C. to 45° C. under vacuum. The yield of crude Compound I was 88%.The crude crystals of compound I (14.54 kg, 25.6 moles) were recrystalized by dissolving the crude compound in a mixture of fine spirit (35 liters; 95:5 ethanol/isopropanol), water with endotoxin control (35 liters) and hydrochloride acid (0.3 liter, 37%), and heating the solution to reflux with agitation. The refluxing solution was cooled and maintained at a temperature of between 74° C. to 77° C., and filtered through a preheated pipe and in-line filter. The apparatus was rinsed with a mixture of fine spirit (95:5 ethanol/isopropanol) and water with endotoxin control maintained at 60° C. to 70° C. and combined with the filtrate. The temperature of the solution thus provided was adjusted and maintained at a temperature between 72° C. and 74° C. and Compound I seed crystals were added. The seeded solution was maintained at this temperature for 15 to 20 minutes and then cooled to a temperature between 0° C. and 5° C. at the rate of 0.5° C. per minute. The seeded solution was maintained at a temperature between 0° C. and 5° C. and agitated for 30 to 40 minutes. At the end of the time the resulting mixture was filtered and washed with a 40:60 mixture of fine spirit (95:5 ethanol/isopropanol)/water with endotoxin control at 0° C. to 5° C. The wet cake was dried under vacuum (150 mbar to 200 mbar) at 35° C. to 40° C. under vacuum. The yield of the compound of Formula I was determined by HPLC to be 97% based on the amount of the S-isomer present in the solids used initially.A second run was carried out in accordance with the foregoing, however, at the end of the reaction period the reaction mixture was extracted with aqueous sodium carbonate solution and the phases were split. The organic phase was added to dilute HCl to provide spontaneous crystallization. In a subsequent run, when spontaneous crystallization did not occur, seed crystals were charged to seed crystal formation. Once crystalline product had precipitated, the product was filtered and the cake washed successively with aliquots of water, a 1:1 mixture (vol.) of toluene:MTBE, and MTBE. The cake thus obtained was dried under vacuum at 40°-45° C. for approximately 8 h.
References:
US2010/87426,2010,A1 Location in patent:Page/Page column 14-15
![2-Oxa-1,8-diazaspiro[5.5]undecan-3-one, 9-[[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]methyl]-9-phenyl-, (9S)-](/CAS/20210305/GIF/1065272-51-6.gif)
1065272-51-6
0 suppliers
inquiry

858102-79-1
12 suppliers
inquiry
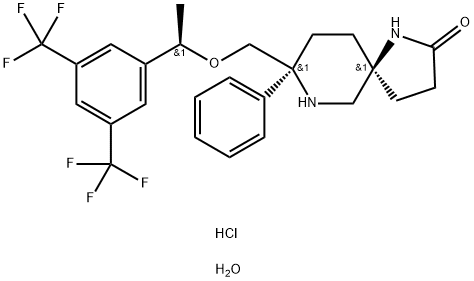
914462-92-3
73 suppliers
$170.00/10mg

858102-79-1
12 suppliers
inquiry
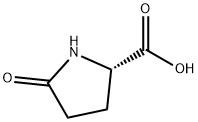
98-79-3
657 suppliers
$5.00/25g

858102-79-1
12 suppliers
inquiry
![(3R,7aS)-3-tert-butyldihydropyrrolo[1,2-c]oxazole-1,5(3H,6H)-dione](/CAS/GIF/171284-84-7.gif)
171284-84-7
28 suppliers
inquiry

858102-79-1
12 suppliers
inquiry