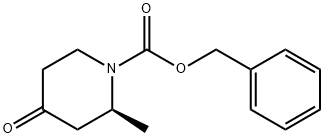
(S)-1-CBZ-2-METHYL-PIPERIDIN-4-ONE synthesis
- Product Name:(S)-1-CBZ-2-METHYL-PIPERIDIN-4-ONE
- CAS Number:921599-74-8
- Molecular formula:C14H17NO3
- Molecular Weight:247.29
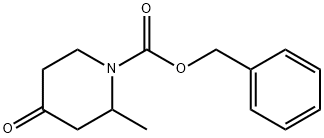
849928-34-3
72 suppliers
$36.00/250mg

921599-74-8
24 suppliers
$125.00/100mg
Yield:921599-74-8 96%
Reaction Conditions:
with Chiralpak AD in methanol;
Steps:
1.3
Step 3. Isolation of benzyl (2S)-2-methyl-4-oxopiperidine-1 -carboxylate (C3). Racemic benzyl 2-methyl-4-oxopiperidine-1-carboxylate (C2) (4.01 kg, 16.2 mol) was dissolved in methanol (80 L) and separated into its enantiomers via chromatography (six Chiralpak AD columns, each 5 cm x 10 cm, 20 μηη, 40 °C; Eluant: methanol; Feed rate 7.2 mL/minute; Eluant rate: 225 mL/minute). The first-eluting enantiomer was the desired (2S) enantiomer, obtained as an amber oil. Yield of benzyl (2S)-2-methyl-4- oxopiperidine-1 -carboxylate (C3): 1 .932 kg, 7.813 mol, 96% with respect to available desired enantiomer. Chiral purity via HPLC analysis: 99.5:0.5 (Column: Chiralpak AD-H, 4.6 mm x 150 mm, 5 μηη; Eluant: 0.1 % trifluoroacetic acid in methanol). {Note: in a similar chiral separation carried out on a different batch of racemic material, conversion to the dimethyl ketal benzyl 4,4-dimethoxy-2-methylpiperidine-1 -carboxylate was observed. This was attributed to slight acidity in the starting material and column packing. A different chiral separation was therefore developed to avoid the presence of alcohol (Column: Chiralpak IA, 20 μηη; Eluant: 60/40 heptane/propan-2-yl acetate). Using six 1 cm x 10 cm columns, a sample of benzyl 2-methyl-4-oxopiperidine-1 -carboxylate (C2) (2.96 g) provided benzyl (2S)-2-methyl-4-oxopiperidine-1-carboxylate (C3) in a 99.5:0.5 ratio with its enantiomer (Chiral purity was assessed via HPLC analysis;Column: Chiralpak IA, 4.6 mm x 250 mm, 5 μηη; Eluant: 60/40 heptane/propan-2-yl acetate). The desired (2S) enantiomer was the first-eluting enantiomer.}
References:
WO2012/172449,2012,A1 Location in patent:Page/Page column 25-26
75-24-1
167 suppliers
$54.50/100ml
185847-84-1
84 suppliers
$20.00/100mg

921599-74-8
24 suppliers
$125.00/100mg

75-16-1
274 suppliers
$12.00/10ml

185847-84-1
84 suppliers
$20.00/100mg

921599-74-8
24 suppliers
$125.00/100mg

501-53-1
408 suppliers
$10.00/1g

921599-74-8
24 suppliers
$125.00/100mg
620-08-6
313 suppliers
$5.00/1g

921599-74-8
24 suppliers
$125.00/100mg