(S)-1-CBZ-AMINO-BUTYL-3-AMINE synthesis
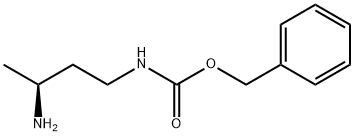
- Product Name:(S)-1-CBZ-AMINO-BUTYL-3-AMINE
- CAS Number:949916-64-7
- Molecular formula:C12H18N2O2
- Molecular Weight:222.28
![Carbamic acid, N-[(3S)-3-[[(1,1-dimethylethoxy)carbonyl]amino]butyl]-, phenylmethyl ester](/CAS/20210305/GIF/949916-62-5.gif)
949916-62-5
1 suppliers
inquiry

949916-64-7
15 suppliers
inquiry
Yield:949916-64-7 75%
Reaction Conditions:
with hydrogenchloride in ethanol at 20; for 3 h;enantioselective reaction;
Steps:
(S)N1(Benzyloxycarbonyl)1,3diaminobutane (V).
2 mL of 6.7 HCl/EtOH was added to a solution of1.0 g (3.1 mmol) (III) in 5 mL of dry Et and after3 h at 20° the reaction mixture was evaporated todryness in vacuo at 20°C. The residue was dissolved in10 mL of dry tOH, poured into 40 mL dry Et2O andleft for a night at -20°C. Separated oil was washedwith dry Et2O (10 mL) and to the residue 4 mL of 2 NaOH and 6 mL of CH2Cl2 were added. Organicphase was separated, H2Olayer was extracted withCH2Cl2 (4 × 4 mL) and the combined organic extractswere washed subsequently with H2O (2 mL), 5 NaCl (3 × 5 mL) and dried (K2CO3). Solvent was distilled off in vacuo and the residue was dried in vacuoover P2O5/KOH to give (V) (0.515 g, 75%) as a viscousoil, Rf 0.44 (B), 1H NMR (CDCl3): 7.35-7.26 (5H, m,65), 5.48 (1H, bs, NHCbz), 5.09 (2H, s,CH265), 3.4-3.2 (2H, m, CbzNHCH2), 3.01-2.92(1H, m, 3CH), 1.62-1.55 (1H, m, NHCH2CH2),1.47-1.38 (1H, m, NHCH2CH2), 1.25-1.22 (2H, m,NH2), 1.20 (3H, d, J 6.5, CH3). 13C NMR (CDCl3):156.55, 136.85, 128.55, 128.09, 77.40, 77.09, 76.77,66.60, 45.56, 39.18, 29.75, 24.90.
References:
Khomutov;Keinanen;Hyvonen;Weisell;Vepsalainen;Alhonen;Kochetkov;Khomutov [Russian Journal of Bioorganic Chemistry,2015,vol. 41,# 5,p. 548 - 553]

501-53-1
409 suppliers
$10.00/1g

949916-64-7
15 suppliers
inquiry