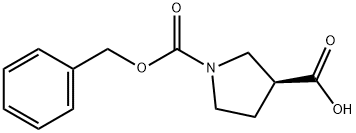
(S)-1-Cbz-pyrrolidine-3-carboxylic acid synthesis
- Product Name:(S)-1-Cbz-pyrrolidine-3-carboxylic acid
- CAS Number:192214-00-9
- Molecular formula:C13H15NO4
- Molecular Weight:249.26
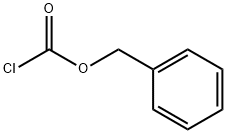
501-53-1
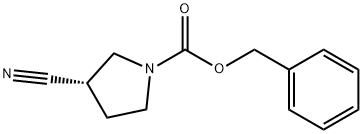
193693-69-5

192214-00-9
Step 1: To a 100 mL round bottom flask was added (S)-1-Cbz-3-pyrrolidinecarbonitrile (2 g, 8.7 mmol) and concentrated hydrochloric acid (20 mL). The mixture was refluxed for 4.5 h before the solvent was removed by vacuum distillation and dried overnight using an oil pump. The crude product was dissolved in a solvent mixture of acetone (20 mL) and deionized water (20 mL). After cooling to 0 °C, sodium carbonate (2.8 g, 26 mmol) was added, followed by slow dropwise addition of benzyl chloroformate (5.5 mL, 9.6 mmol). The reaction mixture was allowed to warm up gradually to room temperature. After 7 hours of reaction, the solvent was removed by vacuum distillation. Deionized water (8 mL) was added and extracted twice with a solvent mixture of hexane:EtOAc = 1:1. The aqueous layer was acidified to pH 2 by the addition of concentrated hydrochloric acid and 0.5 M potassium bisulfate.Subsequently, the aqueous layer was extracted with ethyl acetate (four times), and the organic phase was dried over sodium sulfate and concentrated by vacuum to afford (S)-1-CBZ-3-carboxypyrrolidine (1.46 g, 67% yield), which was used directly in the next step.1H NMR (CDCl3, 400 MHz): δ 7.38-7.29 (m, 5H), 5.14 (d, J = 2.8 Hz, 2H), 3.72-3.43 (m, 4H), 3.17-3.09 (m, 1H), 2.21-2.14 (m, 2H).
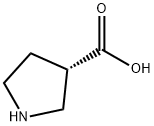
72580-53-1
125 suppliers
$45.00/50mg

501-53-1
409 suppliers
$10.00/1g

192214-00-9
95 suppliers
$28.00/250mg
Yield: 51%
Reaction Conditions:
with sodium hydroxide in water at 0; for 2 h;
Steps:
46
General procedure: To a solution of 103 20a (500mg, 1.616mmol) in 82 MeOH (13mL) 10% 108 Pd/C (179mg) was added and the mixture was allowed to stir for 20h at 25°C under H2 atmosphere. The reaction was filtered on Celite and the filtrate was concentrated under reduced pressure quantitatively providing (R)-pyrrolidine-3-carboxylic acid submitted to the next step without further purification. To a solution of this latter (460mg, 3.995mmol) in 2N 109 NaOH (2mL) 110 benzylchloroformate (740μL, 5.194mmol) and 4N NaOH (1.5mL) were added and the mixture was stirred at 0°C for 2h. The reaction was extracted with diethyl ether. The aqueous layer was adjusted to pH=2 with 10% HCl at 0°C and extracted with EtOAc. The organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography
References:
Ghosh, Arun K.;Brindisi, Margherita;Nyalapatla, Prasanth R.;Takayama, Jun;Ella-Menye, Jean-Rene;Yashchuk, Sofiya;Agniswamy, Johnson;Wang, Yuan-Fang;Aoki, Manabu;Amano, Masayuki;Weber, Irene T.;Mitsuya, Hiroaki [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 19,p. 5114 - 5127]

501-53-1
409 suppliers
$10.00/1g

193693-69-5
59 suppliers
inquiry

192214-00-9
95 suppliers
$28.00/250mg
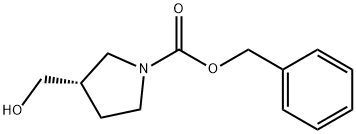
124391-76-0
75 suppliers
$151.41/250mg

192214-00-9
95 suppliers
$28.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

192214-00-9
95 suppliers
$28.00/250mg

193693-69-5
59 suppliers
inquiry

192214-00-9
95 suppliers
$28.00/250mg