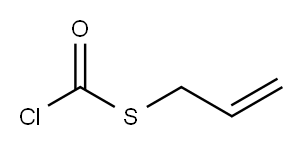
S-2-PROPEN-1-YL ESTER CARBONOCHLORIDOTHIOIC ACID synthesis
- Product Name:S-2-PROPEN-1-YL ESTER CARBONOCHLORIDOTHIOIC ACID
- CAS Number:42068-67-7
- Molecular formula:C4H5ClOS
- Molecular Weight:136.6
Yield:42068-67-7 86.24%
Reaction Conditions:
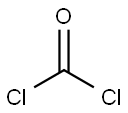
Stage #1: phosgene;prop-2-ene-1-thiol in N,N-dimethyl-formamide;xylene at 0 - 41; for 14 h;Inert atmosphere;
Stage #2: with water in N,N-dimethyl-formamide;xylene at 0 - 5;Product distribution / selectivity;Inert atmosphere;
Steps:
1
65.34 g (0.402 mole) of allyl mercaptan obtained by the process described in Reference Example 1, 2.37 g (0.032 mole, 40% by weight based on the whole amount of N,N-dimethylformamide) of N,N-dimethylformamide and 403.21 g of xylene were charged to a glass reactor equipped with a phosgene gas inlet, a reflux condenser, a thermometer, a stirrer and a jacketed dropping funnel and stirred. Subsequently, nitrogen was introduced into the gaseous phase in the reactor to maintain a nitrogen atmosphere. Then, 41.80 g (0.423 mole) of phosgene was introduced into the reaction solution over 2 hours while maintaining the reaction solution at a temperature of 39 to 41° C. Thereafter, a mixture of 195.31 g of allyl mercaptan (1.207 mole), 3.53 g (0.048 mole, 60% by weight based on the whole amount of N,N-dimethylformamide) of N,N-dimethylformamide was charged to the jacketed dropping funnel, cooled to 0 to 5° C. and dropwise added to the reaction solution over 5 hours while maintaining a temperature of the reaction solution at 39 to 41° C. Simultaneously, 104.50 g (1.056 moles) of phosgene was introduced into the reaction solution over 5 hours. Furthermore, 20.90 g (0.211 mole) of phosgene was introduced into the reaction solution over hour while maintaining the reaction solution at a temperature of 39 to 41° C. Then, the reaction solution was maintained at a temperature of 39 to 41° C. for 3 hours. The reaction solution obtained was cooled to 0 to 5° C., and 264.08 g of water was added to the reaction solution to decompose the unreacted phosgene. The reaction solution was separated to an oil phase and an aqueous phase to obtain 740.76 g of xylene solution of allyl chlorothioformate as an organic phase.The content of allyl chlorothioformate in the solution was 25.60% by weight. The yield of allyl chlorothioformate based on allyl mercaptan was 86.24%. As a result of gas chromatography analysis, the area ratio of the amide compound of the by-product (III) to the amide compound of the chlorothioformate (II) was 0.03%.
References:
US2011/251429,2011,A1 Location in patent:Page/Page column 3

7124-50-7
0 suppliers
inquiry

42068-67-7
4 suppliers
inquiry
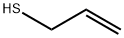
870-23-5
147 suppliers
$70.00/2.5g
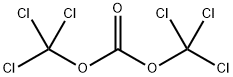
32315-10-9
436 suppliers
$10.00/1g

42068-67-7
4 suppliers
inquiry