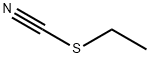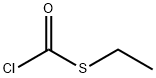
Ethyl chlorothioformate synthesis
- Product Name:Ethyl chlorothioformate
- CAS Number:2941-64-2
- Molecular formula:C3H5ClOS
- Molecular Weight:124.59
Yield:-
Reaction Conditions:
with triethylamine in tetrahydrofuran at -15 - 20; for 0.5 h;
Steps:
4
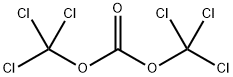
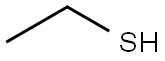
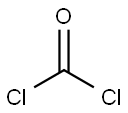
Dissolve ethyl mercaptan (37 mg, 0.6 mmol), triethylamine (61 mg, 0.6 mmol) in tetrahydrofuran (30 ml). Drop triphosgene (180 ml, 0.6 mmol) in tetrahydrofuran (5ml) at -15°C. Allow the reaction to warm up to room temperature and stir for 30 min. Add intermediate 0021 (300 mg, 0.9 mmol). Stir at 500C for 1 h. Distill to remove left solvent. Add dichloromethane (20 ml) and wash with water (20 mix 3). The organic phase was dried with sodium sulfate anhydride. Distill to remove solvent under vacuum. The product 027 (220 mg) was given by column purification with chloroform/methanol.'H- NMR(DMSO-d): 1.23(3H, t), 2.87(2H, m), 3.74(3H, s), 3.84(3H, s), 6.62-7.72(4H, m), 1O.39(1H, s). m/z: 396.01; m.p 200°C-202°C.
References:
CELESTIAL PHARMACEUTICALS (SHENZHEN) LTD.;WELICHEM BIOTECH INC. WO2008/38175, 2008, A2 Location in patent:Page/Page column 41