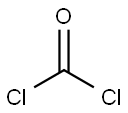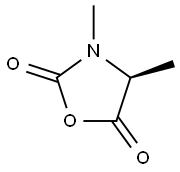
(S)-3,4-Dimethyloxazolidine-2,5-dione synthesis
- Product Name:(S)-3,4-Dimethyloxazolidine-2,5-dione
- CAS Number:58311-53-8
- Molecular formula:C5H7NO3
- Molecular Weight:129.11
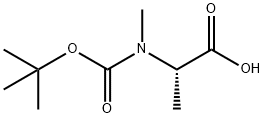
![(2S)-2-[(methoxycarbonyl)(methyl)amino]propanoic acid](/CAS/20180527/GIF/1085528-19-3.gif)
1085528-19-3

58311-53-8
Explosion-proof operation. Synthesis of (S)-3,4-dimethyloxazolidine-2,5-dione: 1 g (6.2 mmol) of the raw material (cas:1085528-19-3) was dissolved in 3 ml of dichloromethane and stirred for 5 min at 20 °C. Subsequently, 500 μl (1.1 eq.) of thionyl chloride was added. The reaction mixture was warmed to 30 °C and maintained at this temperature for 30 min. Upon completion of the reaction, the mixture was concentrated to 1.5 times the original volume and 10 ml of n-heptane was added slowly with continuous stirring. The resulting white solid was cooled to -20°C and stirred at this temperature for 1 hour. The suspension was filtered through a sintered glass funnel and the solid was washed three times with 3 ml of n-heptane. The solid was dried in air for 2 h to give a white acicular product in a yield of 700 mg in 87.4% yield. The structure of the product was confirmed by 1H NMR (DMSO-d6, 400 MHz): δ 1.39 (d, J = 7.5 Hz, 3H); 2.83 (s, 3H); 4.40 (q, J = 7.5 Hz, 1H).
![(2S)-2-[(methoxycarbonyl)(methyl)amino]propanoic acid](/CAS/20180527/GIF/1085528-19-3.gif)
1085528-19-3
13 suppliers
inquiry

58311-53-8
64 suppliers
$45.00/10mg
Yield: 87.4%
Reaction Conditions:
with thionyl chloride in dichloromethane at 20 - 30; for 0.583333 h;
Steps:
2 Preparation of (S)-3,4-dimethyl-1,3-oxazolidine-2,5-dione
Ex. 2 Preparation of (S)-3,4-dimethyl-1,3-oxazolidine-2,5-dione 1 g (6.2 mmol) of the product obtained previously was dissolved in 3 ml of CH2Cl2, stirred for 5 minutes at around 20° C., then treated with SOCl2 (500 μl, 1.1 eq.). The reaction medium was then heated at around 30° C., and the heating was maintained for around 30 min. The reaction medium was then concentrated to around 1.5 volumes, and, still with stirring, 10 ml of n-heptane were added. The white mass thus obtained was then cooled to around -20° C., and stirred for 1 hour at this temperature. The suspension was then filtered through sintered glass, the solid was washed with 3 times 3 ml of n-heptane. After drying in air for 2 hours, the product (700 mg, 87.4%) was obtained in the form of white needles. Structural analyses: 1H NMR (DMSO-d6 at 400 MHz): 1.39 (d, J=7.5 Hz, 3H); 2.83 (s, 3H); 4.40 (q, J=7.5 Hz, 1H).
References:
SANOFI-AVENTIS US2010/113797, 2010, A1 Location in patent:Page/Page column 3-4
16948-16-6
324 suppliers
$6.00/1g

58311-53-8
64 suppliers
$45.00/10mg
3913-67-5
309 suppliers
$6.00/1g
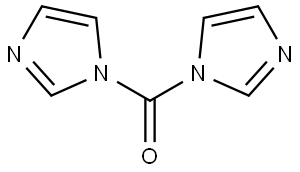
530-62-1
976 suppliers
$6.00/25g

58311-53-8
64 suppliers
$45.00/10mg

3913-67-5
309 suppliers
$6.00/1g
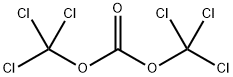
32315-10-9
442 suppliers
$10.00/1g

58311-53-8
64 suppliers
$45.00/10mg