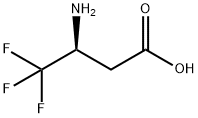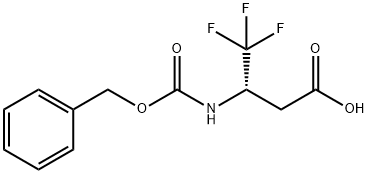
(S)-Cbz-3-Amino-4,4,4-trifluorobutanoic acid synthesis
- Product Name:(S)-Cbz-3-Amino-4,4,4-trifluorobutanoic acid
- CAS Number:1458674-47-9
- Molecular formula:C12H12F3NO4
- Molecular Weight:291.22
![Butanoic acid, 4,4,4-trifluoro-3-[[(phenylmethoxy)carbonyl]amino]-, ethyl ester, (3S)-](/CAS/20210305/GIF/1458674-46-8.gif)
1458674-46-8
0 suppliers
inquiry

1458674-47-9
16 suppliers
inquiry
Yield:1458674-47-9 91%
Reaction Conditions:
with lithium hydroxide monohydrate;water in 1,4-dioxane at 0 - 20;
Steps:
4.3.3 Synthesis of 4 (Scheme 1, route B)
General procedure: To a 1,4-dioxane solution (3mL) of 3 (306mg, 1.00mmol) was added an aqueous solution (3mL) of LiOH·H2O (126mg, 3.00mmol) at 0°C. The mixture was allowed to warm to rt and stirred at the temperature for 1h. The resultant mixture was treated with 3M aqueous hydrochloric acid (1.5mL) and extracted with ethyl acetate (3×3mL). Organic layers combined were dried over anhydrous Na2SO4 and concentrated under reduced pressure to dryness. The resultant residue was dissolved in a mixture of saturated aqueous solution of NaHCO3 (5mL) and water (5mL). The aqueous solution was washed with chloroform (3×5mL), treated with 3M aqueous hydrochloric acid until the pH of the mixture became ca. 3, and extracted with ethyl acetate (3×5mL). Organic layers combined were washed with brine (15mL), dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure to afford 4 as a white solid (266mg, 0.91mmol, 91%). Physical properties of this sample thus obtained were essentially identical to those of 4 obtained by Scheme 1, routeA.
References:
Cho, Joon-Il;Nishizono, Nao;Iwahashi, Nobutaka;Saigo, Kazuhiko;Ishida, Yasuhiro [Tetrahedron,2013,vol. 69,# 44,p. 9252 - 9260]
501-53-1
407 suppliers
$10.00/1g

1458674-47-9
16 suppliers
inquiry