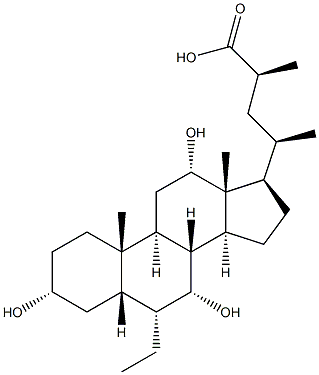
S-EMCA synthesis
- Product Name:S-EMCA
- CAS Number:1199796-29-6
- Molecular formula:C27H46O5
- Molecular Weight:450.6512
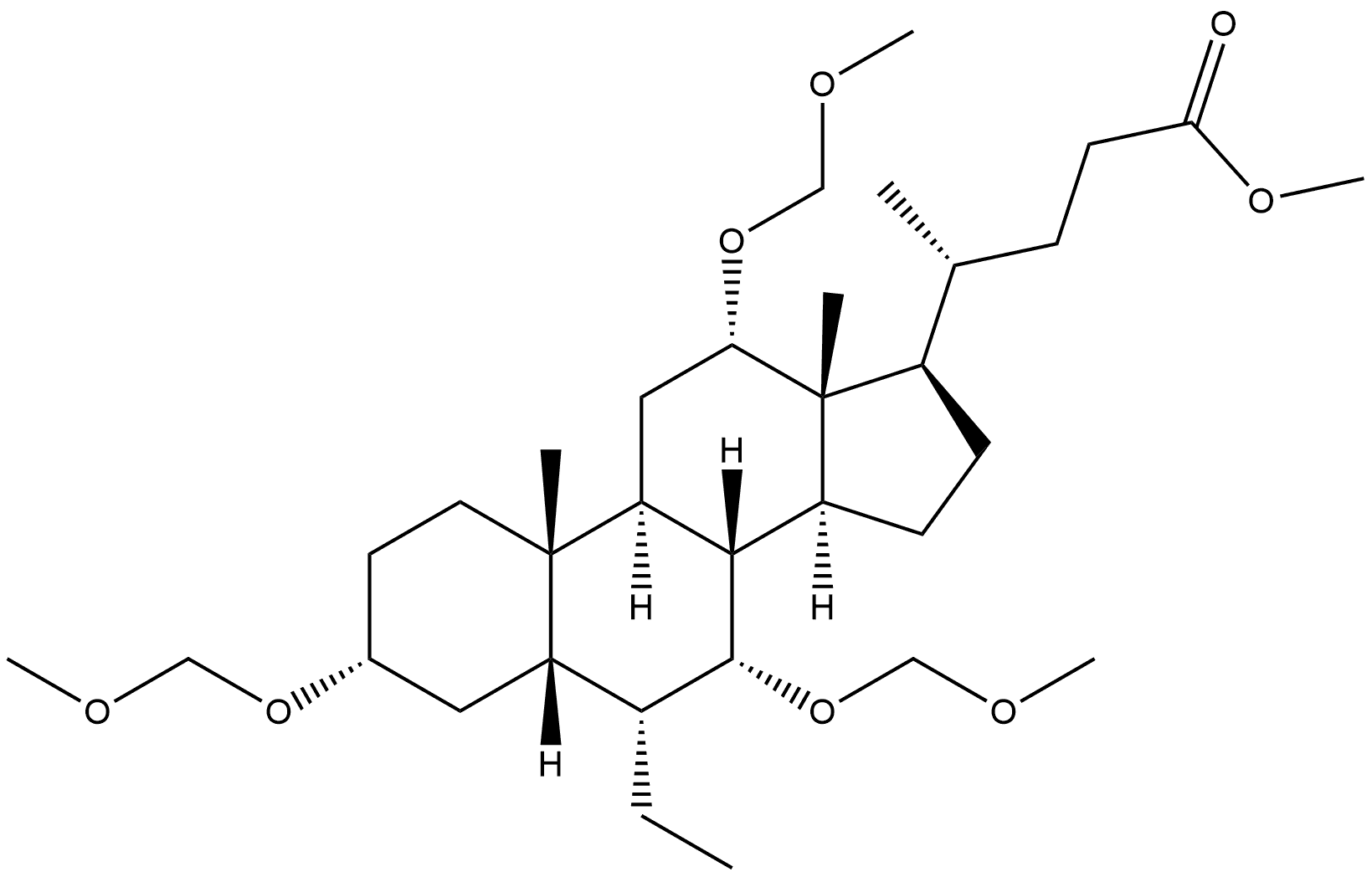
1227639-47-5
0 suppliers
inquiry

74-88-4
356 suppliers
$15.00/10g

1199796-29-6
62 suppliers
$99.00/500μg
Yield:1199796-29-6 41%
Reaction Conditions:
Stage #1:methyl 3α,7α,12α-trimethoxymethyloxy-6α-ethyl-5β-cholan-24-oate with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78;Inert atmosphere;
Stage #2:methyl iodide in tetrahydrofuran;hexane at -78 - 20;
Steps:
6
23(S)-methyl-3α,7α,12α-trihydroxy-6α-ethyl-5β-cholan-24-oic acid (Ih3e); To a solution of diisopropylamine (0.56 ml, 4.026 mmol) in freshly distilled THF (15 ml) cooled at -78° C. and under N2 atmosphere, 11BuLi 2.5N in hexane (1.53 ml, 3.840 mmol) was added dropwise. The reaction was stirred at -78° C. for 30' and then a solution of 3 (350 mg, 0.601 mmol) dissolved in freshly distilled THF (7 ml) was added dropwise. The resulting solution was stirred at -78° for 90'. Iodomethane (0.56 ml, 9.015 mmol) was added, the reaction mixture was stirred at -78° C. for 60', and then slowly warmed to room temperature overnight. The mixture was then concentrated under reduced pressure, and the resulting residue was diluted with H2O (30 ml) and extracted with AcOEt (3×30 ml). The collected organic layers were washed with brine (30 ml), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was then treated with a solution of MeOH/HCl 37% (20 ml, 20:1 vol/vol) at 45° for 8 h. The mixture was concentrated under reduced pressure, and the resulting residue was diluted with H2O (30 ml) and extracted with AcOEt (3×30 ml). The combined organic layers were washed with brine (100 ml), dried over anhydrous Na2SO4, and concentrate under reduced pressure. The resulting residue was treated with a solution of NaOH 10% in MeOH (15 ml) at 45° C. for 24 h. The mixture was then concentrated under reduced pressure, and the resulting residue was diluted with H2O (20 ml), washed with iPr2O (3×15 ml), acidify with HCl 3N, and finally extracted with CHCl3 (3×20 ml). The organic layers were washed with brine (100 ml), dried over anhydrous Na2SO4, and concentrated. The resulting residue was purified by medium pressure chromatography (column: “RP-1 8 Lobar B”, MeOH/H2O from 5:5 to 6:4, 50 psi) to give 4 (47 mg, 41%).Mp: 195-197° C.1H-NMR (CDCl3+CD3OD) δ: 0.63 (3H, s, 18-CH3), 0.84-0.88 (6H, m, 19-CH3+CH3CH2), 0.98 (3H, d, J=6.60 Hz, 21-CH3), 1.10 (3H, d, J=6.80 Hz, CH(CH3)COOH), 2.61 (m, 1H, CH(CH3)COOH), 3.35 (1H, m. 3-CH), 3.65 (1H, m, 7-CH), 3.92 (1H, m, 12-CH).13C-NMR (CDCl3+CD3OD) δ: 11.51, 12.34, 17.52, 19.19, 22.09, 22.67, 23.11, 26.65, 27.40, 28.05, 29.83, 33.31, 34.56, 35.06, 35.40, 38.67, 39.90, 41.11, 41.39, 41.69, 45.10, 46.39, 47.32, 70.65, 71.79, 72.90, 182.07.
References:
Intercept Pharmaceuticals, Inc. US2010/152151, 2010, A1 Location in patent:Page/Page column 28