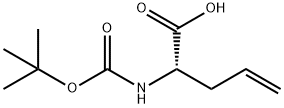
(S)-N-Boc-allylglycine synthesis
- Product Name:(S)-N-Boc-allylglycine
- CAS Number:90600-20-7
- Molecular formula:C10H17NO4
- Molecular Weight:215.25
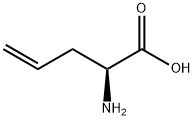
16338-48-0
256 suppliers
$11.00/250mg

24424-99-5
870 suppliers
$13.50/25G

90600-20-7
137 suppliers
$15.00/100mg
Yield:90600-20-7 100%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water at 0 - 20; for 18 h;
Steps:
13.1
Stage 1; - (2S)-2-[(fert-Butoxycarbonyl)amino]pent-4-enoic acidTo a solution of (2S)-2-aminopent-4-enoic acid (1.0Og, 8.70mmol) in 1 M NaOH (2OmL) and dioxane (1OmL) at O0C was added BOC2O (2.28g, 10.5mmol). The reaction mixture was allowed to warm to RT and stirred for an additional 18 hours. The pH was checked and adjusted to basic when necessary. The reaction mixture was concentrated under reduced pressure and the aqueous phase washed with Et2O (2 x 1OmL) to remove the excess BOC2O. The aqueous phase was acidified to pH2 with 2M H2SO4 and extracted with EtOAc (4 x 2OmL) while saturating the aqueous each time with sodium chloride. The combined organic layers were dried (MgSO4) and concentrated under reduced pressure to afford the product (2.2g, 100% yield). ESMS m/z: 238 [M+Na]+.
References:
CHROMA THERAPEUTICS LTD. WO2008/50096, 2008, A1 Location in patent:Page/Page column 50
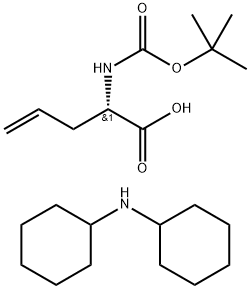
143979-15-1
143 suppliers
$9.00/1g

90600-20-7
137 suppliers
$15.00/100mg
![4-Pentenoic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]-, ethyl ester](/CAS/20210111/GIF/135722-56-4.gif)
135722-56-4
0 suppliers
inquiry

90600-20-7
137 suppliers
$15.00/100mg

24424-99-5
870 suppliers
$13.50/25G

90600-20-7
137 suppliers
$15.00/100mg
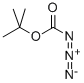
1070-19-5
12 suppliers
inquiry

16338-48-0
256 suppliers
$11.00/250mg

90600-20-7
137 suppliers
$15.00/100mg