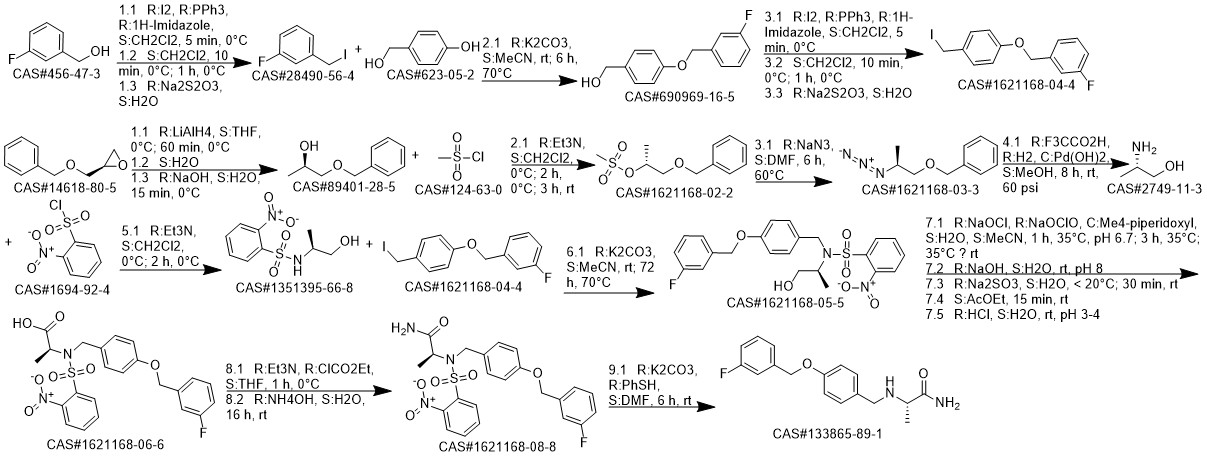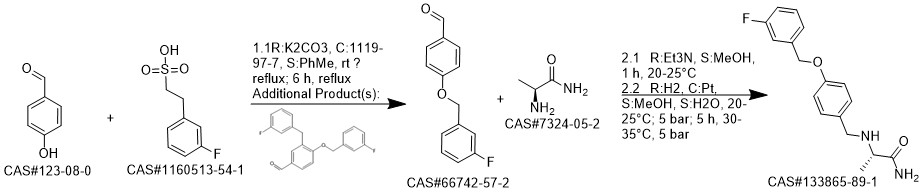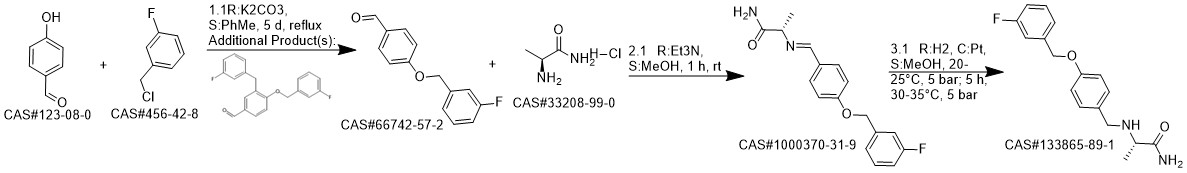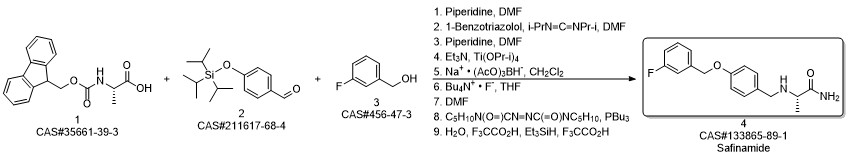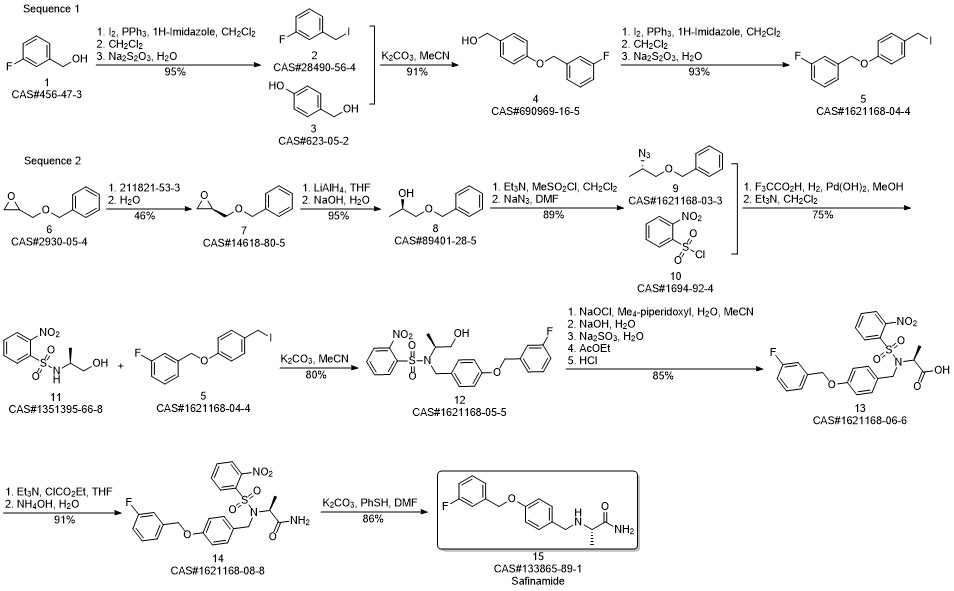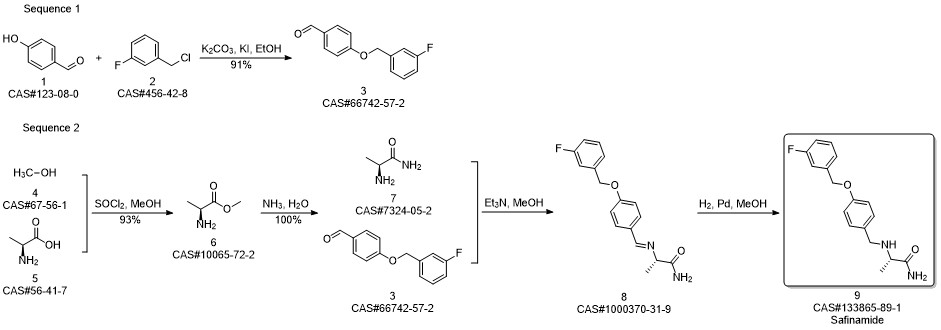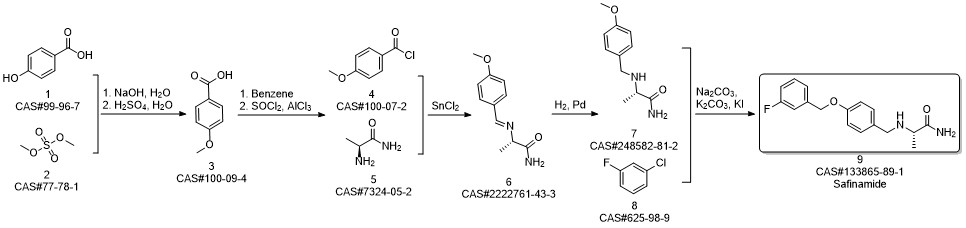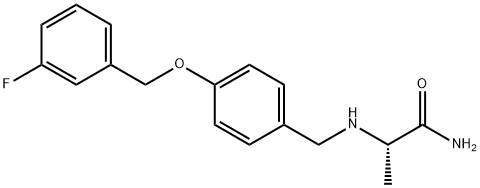
Safinamide synthesis
- Product Name:Safinamide
- CAS Number:133865-89-1
- Molecular formula:C17H19FN2O2
- Molecular Weight:302.34
Park, Ki Duk; Yang, Xiao-Fang; Dustrude, Erik T.; Wang, Yuying; Ripsch, Matthew S.; White, Fletcher A.; Khanna, Rajesh; Kohn, Harold. Chimeric agents derived from the functionalized amino acid, lacosamide, and the α-aminoamide, safinamide: evaluation of their inhibitory actions on voltage-gated sodium channels, and antiseizure and antinociception activities and comparison with lacosamide and safinamide. Division of Chemical Biology and Medicinal Chemistry, UNC Eshelman School of Pharmacy. (ACS Chemical Neuroscience Volume 6 Issue 2)
1000370-31-9
9 suppliers
inquiry

133865-89-1
272 suppliers
$15.00/100mg
Yield:133865-89-1 96%
Reaction Conditions:
with potassium borohydride in methanol;
Steps:
2 Example 2
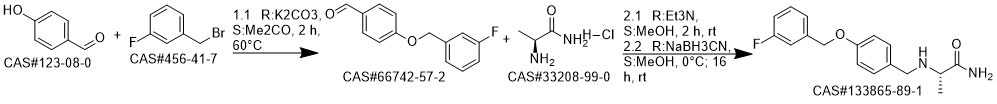
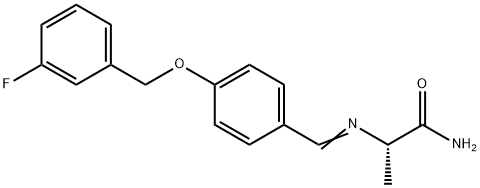
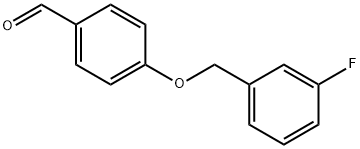
A solution of L-alanine amide hydrochloride 84. 41 g (0.68 mol) was added to the reaction flask,Triethylamine 171 · 42 g (l. 69 mol)Methanol 1300ml, room temperature stirring 30min,Then add 4- (3-fluorobenzyloxy) benzaldehyde 130. llg (0. 56 mol) and stirred at room temperature for 2 h.Then add KBH4 182. 71g (3. 39mol) and stir well overnight.After completion of the reaction, the reaction solution was concentrated to dryness,Add 1300ml water room temperature stirring 1h, pumping filter,40 ° C in vacuo for 4 h to give a white solid (S) -2- [4- (3-fluorobenzyloxy)Benzylamino] propanamide 163. 92 g, yield: 96.0%. HPLC purity: 99.04%.
References:
CN106565520,2017,A Location in patent:Paragraph 0016
66742-57-2
177 suppliers
$7.00/250mg
33208-99-0
390 suppliers
$6.00/5g

133865-89-1
272 suppliers
$15.00/100mg
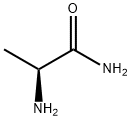
7324-05-2
41 suppliers
$75.00/250mg

66742-57-2
177 suppliers
$7.00/250mg

133865-89-1
272 suppliers
$15.00/100mg
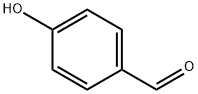
123-08-0
965 suppliers
$5.00/10g

133865-89-1
272 suppliers
$15.00/100mg
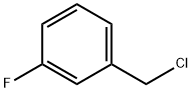
456-42-8
335 suppliers
$5.00/5g

133865-89-1
272 suppliers
$15.00/100mg