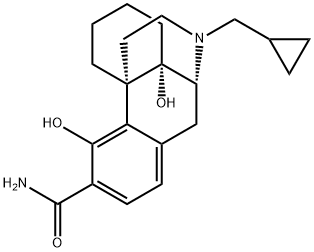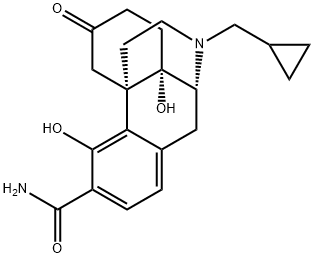
Samidorphan synthesis
- Product Name:Samidorphan
- CAS Number:852626-89-2
- Molecular formula:C21H26N2O4
- Molecular Weight:370.44

421552-35-4
7 suppliers
inquiry

852626-89-2
8 suppliers
inquiry
Yield:852626-89-2 90%
Reaction Conditions:
with ammonium chloride;zinc in ethanol at 60 - 63;Inert atmosphere;
Steps:
A method for converting Compound-A to Compound-B is disclosed in U.S. Pat. No. 7,262,298 which is adopted as described below. A reaction vessel under inert atmosphere was charged with 200 proof ethanol (52.5 L) and Compound-A (3500 g). Ammonium chloride (NH4Cl, 1776 g) and Zinc dust, <10 micron (2234 g) were added and the reaction mixture was heated to 60-63° C. Upon reaction completion as judged by HPLC, reaction mixture was cooled to 18° C. to 22° C., filtered, and concentrated under vacuum to a minimum stirrable volume. Two co-evaporations with 2-Me-THF (2×15 L) to a minimum stirrable volume. The reaction mixture was cooled to 15° C. to 18° C. and 2-Me-THF was added followed by aqua ammonia to adjust the pH of the reaction mixture to 9.0-10.0. The reactor was charged with water (60 L) and MTBE (7 L). The layers were separated and the aqueous layer was further extracted with 2-Me-THF (15 L, 4.5 vol.)/MTBE (3.5 L). The combined organic layers were washed with a brine solution (35 L, 10 vol.). The organic layer was dried with magnesium sulfate, followed by addition of charcoal. The solution was filtered and treated with 3-mercaptopropyl silica gel (Si-Thiol). Remove the solids and concentrate the filtrate under vacuum to a minimum stirrable volume. Two co-evaporations were performed with MTBE (4 vol.) followed by two co-evaporations with Heptanes (4 vol.) to a minimum stirrable volume. The reaction mixture was cooled to 15-18° C. and charged with Heptanes (7 L). The suspension was cooled to <10° C. and stirred for minimum of 1.5 hours. The suspension was filtered, washed with heptanes and dried in a vacuum oven at 30-35° C. to give Compound-B (3090 g, 90% yield). Elemental Analysis: C, -68.35%; H, -7.06%; N, -7.47%. (Theory: 68.09, 7.07, 7.56)H1NMR (400 MHz, CDCl3): 13.2 (1H, s); 7.12 (1H, d, 8.2 Hz), 6.50 (1H, d, 9 Hz); 6.10 (2H, br s); 4.70 (1H, s); 4.05 (1H, d, 11.3 Hz); 3.12 (1H, d, 6.3 Hz); 3.00-2.95 (2H, m); 2.90-2.77 (2H, m), 2.67-2.62 (1H, m); 2.36 (2H, d, 6.4 Hz); 2.2-1.8 (5H, m); 1.75-1.68 (1H, m); 0.9-0.8 (1H, m); 0.58-0.51 (2H, m); 0.15-0.10 (2H, m).
References:
US2012/10412,2012,A1 Location in patent:Page/Page column 20-21
16676-32-7
6 suppliers
inquiry

852626-89-2
8 suppliers
inquiry
![Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-14-hydroxy-3-[[(trifluoromethyl)sulfonyl]oxy]-, cyclic 1,2-ethanediyl acetal, (5α)-](/CAS/20240320/GIF/263713-00-4.gif)
263713-00-4
1 suppliers
inquiry

852626-89-2
8 suppliers
inquiry
![Morphinan-3-carbonitrile, 17-(cyclopropylmethyl)-4,5-epoxy-6,6-[1,2-ethanediylbis(oxy)]-14-hydroxy-, (5α)-](/CAS/20210305/GIF/1354216-15-1.gif)
1354216-15-1
3 suppliers
inquiry

852626-89-2
8 suppliers
inquiry