Silane, (4-iodo-1-butyn-1-yl)trimethyl- synthesis
- Product Name:Silane, (4-iodo-1-butyn-1-yl)trimethyl-
- CAS Number:41423-29-4
- Molecular formula:C7H13ISi
- Molecular Weight:252.17
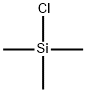
2117-12-6
71 suppliers
$7.00/1g

41423-29-4
3 suppliers
inquiry
Yield:41423-29-4 96%
Reaction Conditions:
with 1H-imidazole;iodine;triphenylphosphine in tetrahydrofuran at 0 - 20; for 2 h;
Steps:
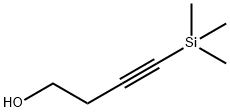
(4-Iodo-but-1-yn-1-yl)trimethylsilane (45)
Following a slightly modified procedure,2 triphenylphosphine (PPh3, 37.9 g, 145 mmol, 1.00 equiv.) wasadded to a cooled solution of 4-(trimethylsilyl)but-3-yn-1-ol (44) (20.6 g, 145 mmol, 1.00 equiv.) intetrahydrofuran (545 mL) at 0 °C. Upon dissolution, imidazole (9.84 g, 145 mmol, 1.00 equiv.) wasadded, followed by iodine (I2, 36.7 g, 145 mmol, 1.00 equiv.). The resulting mixture was then allowed towarm to room temperature and was stirred for 2 hours. It was then diluted with diethyl ether (400 mL)and washed with 10% aqueous sodium thiosulfate (400 mL). The aqueous layer was extracted withadditional portions of diethyl ether (2 x 150 mL) and the combined organic layers were washed withbrine (400 mL), dried over magnesium sulfate, filtered and concentrated in vacuo. The resulting whitesuspension was filtered through a plug of silica, eluting with pentane, to afford pure (4-iodo-but-1-yn-1-yl)trimethylsilane (45) (34.9 g, 138 mmol, 96% yield) as a colorless oil.
References:
Tessier, Romain;Ceballos, Javier;Guidotti, Nora;Simonet-Davin, Raphael;Fierz, Beat;Waser, Jerome [Chem,2019,vol. 5,# 8,p. 2243 - 2263] Location in patent:supporting information

86994-11-8
0 suppliers
inquiry

41423-29-4
3 suppliers
inquiry
![Silane, trimethyl[[4-(trimethylsilyl)-3-butyn-1-yl]oxy]-](/CAS/20200611/GIF/58434-99-4.gif)
58434-99-4
4 suppliers
inquiry

41423-29-4
3 suppliers
inquiry