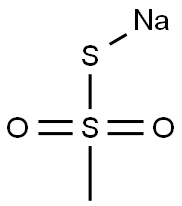
sodium methanethiosulphonate synthesis
- Product Name:sodium methanethiosulphonate
- CAS Number:1950-85-2
- Molecular formula:CH3NaO2S2
- Molecular Weight:134.1531
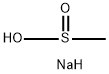
20277-69-4

1950-85-2
The general procedure for the synthesis of sodium sulfur methanesulfonate from sodium methanesulfite was as follows: a mixture of sodium methanesulfonate (5.43 g, 53 mmol) and sulfur powder (1.666 g, 52 mmol) in anhydrous methanol (310 mL) was heated and refluxed for 20 minutes until the sulfur was almost completely dissolved. The reaction mixture was filtered while hot and the filtrate was concentrated to dryness. The resulting off-white solid was stirred with a small amount of anhydrous ethanol at room temperature, filtered and concentrated again. The grinding process was repeated until the 1H NMR spectrum of the white residue showed no sodium methanethiosulfonate residue. All the filtrates were combined and evaporated to dryness to give sodium thiomethanesulfonate (5.40 g, 77% yield) as fine white acicular crystals; melting point 271-272°C (literature value: 272-273.5°C, G.L. Kenyon, T.W. Bruice, Methods Enzymol. 1977, 47, 407-430); 1H NMR ( 200 MHz, CDCl3) δ 3.18 (s, 3H, CH3); Calculated elemental analysis (C8H5NaO2S2): C 8.95, H 2.25; Measured values: C 8.86, H 2.55. An improved synthetic method for NaMTS has been successfully validated, which achieves a high yield synthesis of NaMTS through the reflux reaction of sodium sulfite with sulfur in methanol (Scheme 16, Figure 19), avoiding the cumbersome by-product isolation step in the conventional Na2SZMe3SiCl method. Although a small amount of unknown by-products was generated during the reaction, they were easily separated from NaMTS.
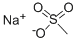
2386-57-4
233 suppliers
$13.00/25g

1950-85-2
71 suppliers
$9.00/250mg
Yield:-
Reaction Conditions:
with hydrogen sulfide in methanol
Steps:
2.2 2.
2. Sodium Methanethiosulfonate A mixture of sodium methanesulfonate (1 gram, 9.8 mmol) and sulfur (312 mg, 9.75 mmol) in methanol (60 ml) was heated under reflux for 3 hours by which point all sulfur had dissolved. The mixture was then filtered, and the filtrate was concentrated in vacuo to dry to give a white solid. 1H NMR (300 MHz, D2O)H 3.375 (3H, s) IR: 1322.9 cm-1, 1201.5 cm-1, 1097.3 cm-1, 977.7 cm-1, 769.3 cm-1
References:
Cooper, Matthew;Li, Xin US2009/275721, 2009, A1
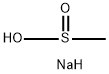
20277-69-4
282 suppliers
$10.00/1g

1950-85-2
71 suppliers
$9.00/250mg

124-63-0
0 suppliers
$10.00/5g

1950-85-2
71 suppliers
$9.00/250mg