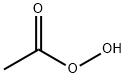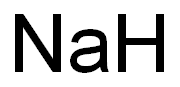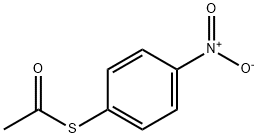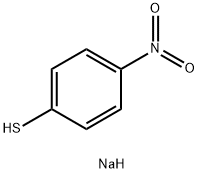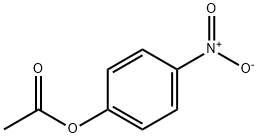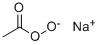
SODIUMPERACETATE synthesis
- Product Name:SODIUMPERACETATE
- CAS Number:64057-57-4
- Molecular formula:C2H4O3.Na
- Molecular Weight:0
102-76-1
677 suppliers
$5.00/25g
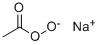
64057-57-4
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with dihydrogen peroxide;sodium hydroxide in water; pH=12 - 12.4;Reagent/catalyst;
Steps:
5
Example 5 Generation of Singlet Oxygen Using Bulk Chemical Precursors (0180) An generation system from FIG. 1 and associated method from FIG. 2 was used in the present example to show an exemplar of producing a singlet oxygen precursor formulation using bulk chemical precursors. A 30 g/L aqueous hydrogen peroxide solution 102 is pH adjusted with sodium hydroxide alkali concentrate 104 to pH 12.0 to 12.4, using approximately 50 g sodium hydroxide per liter of 30 g/L hydrogen peroxide. The resulting alkaline hydrogen peroxide solution is mixed 120(1) and reacted with an acetyl donor 106 of triacetin in a ratio of 128 g triacetin per 1 liter alkaline hydrogen peroxide solution. The resulting alkaline peracid concentrate 122′ will contain approximately 65 g/L peroxyacetic acid and 0.9 g/L hydrogen peroxide assuming 97% conversion of the hydrogen peroxide to peroxyacetic acid. The resulting alkaline peracid concentrate 122′ will also contain about 54 g/L glycerol byproduct 124. (0181) The peroxyacetic acid concentrate is then diluted to its point of use concentration before or during pH adjustment to minimize losses resulting from accelerated peroxyacetic acid decomposition at higher concentrations when in its activated pH range. In the present example, the above peroxyacetic acid concentrate is diluted to 1.5 g/L, a dilution factor of 41.5 times. The peroxyacetic acid solution is diluted with 40.5 L of make up water 108 (fresh water or salt water) and then mixed with an acid concentrate 112 necessary for adjusting the pH to activate singlet oxygen evolution, where an initial pH range is between pH 8 and pH 9. For example, 12 g hydrochloric acid (100% base) is added per 1 L of concentrate. Additionally, other additives may be added to the solution by combining them with water 108 used to dilute alkaline peracid concentrate 122′, for example additives such as: sodium or calcium chloride, tetrasodium pyrophosphate, sodium lauryl sulfate and glycerol. (0182) For the above exemplary singlet oxygen precursor formulation the hydrogen peroxide stock solution, alkali types, and acid types and other additives including salts, surfactants, co-solvents, stabilizers, and emulsifiers can be substituted with compatible alternatives known in the art to accommodate specific application requirements. The resulting singlet oxygen reactive oxygen species output 116 may then be used in the form of a liquid, an ice, a foam, an emulsion, a microemulsion or an aerosol applied by means such as injection, flooding, spraying, circulation or by any other means of conveying a fluid. (0183) The above example 5 may also be implemented using the system of FIG. 3 and method of FIG. 4, without diluting the alkaline peracid concentrate 122′.
References:
US2016/318778,2016,A1 Location in patent:Paragraph 0180-0183