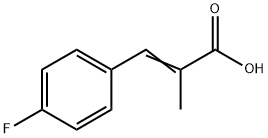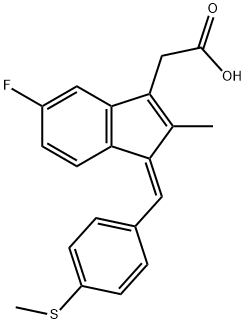
SULINDAC SULFIDE synthesis
- Product Name:SULINDAC SULFIDE
- CAS Number:49627-27-2
- Molecular formula:C20H17FO2S
- Molecular Weight:340.41
38194-50-2
324 suppliers
$11.00/1000mg

49627-27-2
105 suppliers
$28.00/1mg
Yield:49627-27-2 100%
Reaction Conditions:
with titanium tetrachloride;zinc in tetrahydrofuran;dichloromethane; for 2.5 h;Cooling with acetone-dry ice;Inert atmosphere;
Steps:
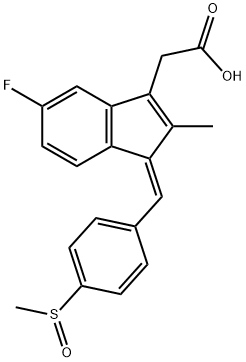
(Z)-2-(5-Fluoro-2-methyl-1-(4-(methylthio)benzylidene)-1H-inden-3-yl)acetic acid (sulindac sulfide), 44
Titanium (IV) chloride (1.0 M solution in CH2Cl2, 8.4 mmol, 8.4 mL) was added to a suspension of zinc dust (1.1 g, 16.8 mmol) in tetrahydrofuran (20 mL) cooled in an ice/acetone bath and the resulting suspension stirred for 10 min. A suspension of sulindac 1 (1.0 g, 2.8mmol) in dichloromethane (20 mL) was added dropwise via syringe pump over 0.5 h and the resultant suspension stirred for 2 h, maintaining cooling. The suspension was filtered through a pad of Celite and the resulting solution was quenched with aq. HCl solution (3 N, 100mL), the solution was extracted with dichloromethane (3 × 60 mL) and the organics combined and dried over Na2SO4, filtered and the solvent removed under reduced pressure to yield (Z)-2-(5-fluoro-2-methyl-1-(4-(methylthio)benzylidene)-1H-inden-3-yl)acetic acid, sulindac sulfide 4 (952 mg, 1.00 mmol, quant.) as a yellow solid, which was used without any further purification: m.p. 186-188 °C, [Lit.4 186-190 °C]; νmax (thin film)/cm-1 2926, 1702, 1557, 1601, 1491, 1048; 1H NMR (500 MHz; CDCl3) δ 7.44 (2H, d, J 8.2 Hz, CH-2′,6′), 7.36 (1H, dd, J 8.4, 5.3 Hz, CH-7), 7.29 (2H, d, J 8.2 Hz, CH-3′,5′), 7.15 (1H, s, CH-α), 6.88 (1H, dd, J 8.9, 2.1 Hz, CH-6), 6.58 (1H, ddd, J 8.9, 8.8, 2.1 Hz, CH-4), 3.59 (2H, s, 3-CH2), 2.55 (3H, s, SCH3) and 2.20 (3H, s, 2-CH3); 13C NMR (100 MHz; CDCl3) δ 175.3 (quat., carboxyl), 163.1 (quat., d, J 246.1 Hz, C-5), 146.2 (quat., d, J 8.4 Hz, C-8), 140.0 (quat., C-1), 139.2 (quat., C-4′), 138.8 (quat., C-2), 132.9 (quat., C-3), 130.2 (quat., C-1′), 130.0 (CH, C-α), 129.9 (CH × 2, C-2′,6′), 129.7 (quat., C-9), 125.9 (CH × 2, C-3′,5′), 123.8 (CH, d, J 8.9 Hz, C-7), 110.7 (CH, d, d, J 22.7 Hz, C-6), 105.7 (CH, d, J 24.3 Hz, C-4), 31.3 (CH2, 3-CH2), 15.4 (CH3, SCH3) and 10.6 (CH3, 2-CH3); 19F {1H} (376 MHz; CDCl3) -114.2 (CF); m/z (ES+) 363 ([M+Na]+, 100%); HRMS m/z (ES)+ calcd. for C20H17FNaO2S [M+Na]+ requires 363.0831, found 363.0839; CHN Anal. calcd. for C20H17FO2S: C, 70.57; H, 5.03. Found C, 70.61; H, 5.11. The data were in agreement with the literature values.4
References:
Nortcliffe, Andrew;Ekstrom, Alexander G.;Black, James R.;Ross, James A.;Habib, Fouad K.;Botting, Nigel P.;O'Hagan, David [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 2,p. 756 - 761] Location in patent:supporting information

53077-50-2
1 suppliers
inquiry
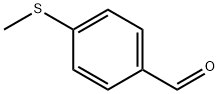
3446-89-7
236 suppliers
$14.00/1g

49627-27-2
105 suppliers
$28.00/1mg

61812-45-1
2 suppliers
inquiry

146857-81-0
0 suppliers
inquiry
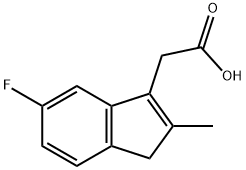
32004-66-3
119 suppliers
$22.00/250mg

49627-27-2
105 suppliers
$28.00/1mg
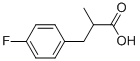
22138-73-4
40 suppliers
$45.00/10mg

49627-27-2
105 suppliers
$28.00/1mg

61812-45-1
2 suppliers
inquiry