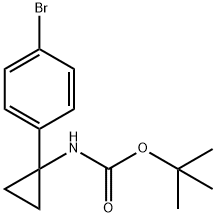
t-Butyl 1-(4-bromophenyl)cyclopropylcarbamate synthesis
- Product Name:t-Butyl 1-(4-bromophenyl)cyclopropylcarbamate
- CAS Number:360773-84-8
- Molecular formula:C14H18BrNO2
- Molecular Weight:312.2

24424-99-5
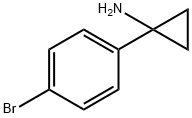
345965-54-0

360773-84-8
The general procedure for the synthesis of tert-butyl [1-(4-bromophenyl)-cyclopropyl]carbamate from 1-(4-bromophenyl)cyclopropan-1-amine and di-tert-butyl dicarbonate was as follows: in a 100-mL round-bottom flask, 1-(4-bromophenyl)cyclopropan-1-amine (1 g, 4.48 mmol, 1.00 equiv) and di-tert-butyl dicarbonate (3.1 g, 13.49 mmol , 3.01 eq.) were dissolved in tetrahydrofuran (20 mL). Subsequently, an aqueous solution of sodium bicarbonate (5 g, 59.5 mmol, 13.29 eq.) was slowly added to the reaction system (10 mL) at room temperature. The reaction mixture was stirred overnight at room temperature. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (3 x 10 mL), the organic layers were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography using ethyl acetate/petroleum ether (10% to 50% gradient) as eluent to afford tert-butyl [1-(4-bromophenyl)-cyclopropyl]carbamate (1.47 g, 99%) as a light yellow solid. Mass spectrometry (MS) analysis showed: m/z = 255.7 [M+H-56]+.

24424-99-5
870 suppliers
$13.50/25G

345965-54-0
93 suppliers
$65.00/100mg

360773-84-8
79 suppliers
$29.00/250mg
Yield:360773-84-8 99%
Reaction Conditions:
with sodium carbonate in tetrahydrofuran;water at 20;
Steps:
53 tert-Butyl N-[1-(4-bromophenyl)cyclopropyl]carbamate
tert-Butyl N-[1-(4-bromophenyl)cyclopropyl]carbamate
In a 100-mL round bottom flask, 1-(4-bromophenyl)cyclopropan-1-amine (1 g, 4.48 mmol, 1.00 equiv) and di-tert-butyl dicarbonate (3.1 g, 13.49 mmol, 3.01 equiv) were mixed in tetrahydrofuran (20 mL), to which was added a solution of sodium bicarbonate (5 g, 59.5 mmol, 13.29 equiv) in water (10 mL) at room temperature.
The resulting solution was stirred overnight at room temperature.
After the reaction was done, the reaction mixture was extracted with ethyl acetate (3*10 mL) and the organic layers were combined, dried over sodium sulfate and concentrated under reduced pressure.
The residue was purified in a silica gel column eluting with ethyl acetate in petroleum ether (10% to 50% gradient) to afford tert-butyl N-[1-(4-bromophenyl)cyclopropyl]carbamate (1.47 g, 99%) as light yellow solid. MS: m/z=255.7 [M+H-56]+.
References:
Merck Patent GmbH;GAILLARD, Pascale;SEENISAMY, Jeyaprakashnarayanan;LIU-BUJALSKI, Lesley;CALDWELL, Richard D.;POTNICK, Justin;QIU, Hui;NEAGU, Constantin;JONES, Reinaldo;WON, Annie Cho;GOUTOPOULOS, Andreas;SHERER, Brian A.;JOHNSON, Theresa L.;GARDBERG, Anna US2016/96834, 2016, A1 Location in patent:Paragraph 0390
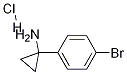
952289-92-8
42 suppliers
$53.00/250mg

24424-99-5
870 suppliers
$13.50/25G

360773-84-8
79 suppliers
$29.00/250mg
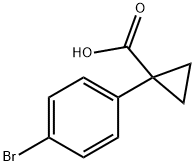
345965-52-8
169 suppliers
$7.00/250mg

75-65-0
787 suppliers
$10.00/10ml

360773-84-8
79 suppliers
$29.00/250mg

345965-52-8
169 suppliers
$7.00/250mg

24424-99-5
870 suppliers
$13.50/25G

360773-84-8
79 suppliers
$29.00/250mg

133017-10-4
0 suppliers
inquiry

360773-84-8
79 suppliers
$29.00/250mg