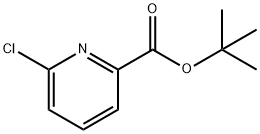
t-Butyl 6-chloro-2-pyridinecarboxylate synthesis
- Product Name:t-Butyl 6-chloro-2-pyridinecarboxylate
- CAS Number:1280786-59-5
- Molecular formula:C10H12ClNO2
- Molecular Weight:213.66
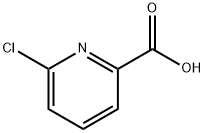
4684-94-0
330 suppliers
$5.00/1g

75-65-0
782 suppliers
$10.00/10ml

1280786-59-5
29 suppliers
$60.00/100mg
Yield:1280786-59-5 96%
Reaction Conditions:
with pyridine;p-toluenesulfonyl chloride at 20;
Steps:
tert-Butyl 6-chloropyridine-2-carboxylate
tert-Butyl 6-chloropyridine-2-carboxylate
6-Chloropicolinic acid (80.0 g, 0.51 mol) was dissolved in pyridine (300 ml) and tert-butanol (1.60 l), then p-toluenesulphonyl chloride (194 g, 1.02 mol) was added in portions.
The reaction mixture was stirred at room temperature overnight.
After addition of saturated aqueous sodium hydrogencarbonate solution, the mixture was stirred at room temperature for 1 h.
The reaction mixture was concentrated to a volume of 1.50 l and diluted with heptane (500 ml), ethyl acetate (500 ml) and water (500 ml).
The organic phase was removed, washed with saturated aqueous sodium chloride solution, dried over magnesium sulphate and filtered, and the filtrate was concentrated. 104 g (96% of theory) of the title compound were obtained.
1H-NMR (400 MHz, CDCl3) δ [ppm]=1.61 (s, 9H), 7.45-7.48 (dd, 1H), 7.74-7.77 (t, 1H), 7.93-7.95 (dd, 1H).
References:
US2019/160048,2019,A1 Location in patent:Paragraph 0829-0831
865-47-4
713 suppliers
$14.00/25ML
80099-98-5
42 suppliers
$45.00/25mg

1280786-59-5
29 suppliers
$60.00/100mg

4684-94-0
330 suppliers
$5.00/1g

1280786-59-5
29 suppliers
$60.00/100mg