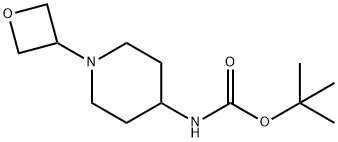
tert-Butyl 1-(oxetan-3-yl)piperidin-4-ylcarbamate synthesis
- Product Name:tert-Butyl 1-(oxetan-3-yl)piperidin-4-ylcarbamate
- CAS Number:1228948-05-7
- Molecular formula:C13H24N2O3
- Molecular Weight:256.34
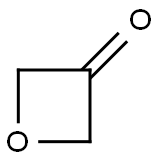
6704-31-0
322 suppliers
$6.00/1g
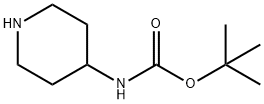
73874-95-0
443 suppliers
$10.00/250mg

1228948-05-7
27 suppliers
$305.00/1g
Yield:1228948-05-7 78%
Reaction Conditions:
Stage #1: 3-oxacyclobutanone;piperidin-4-ylcarbamic acid tert-butyl esterwith glacial acetic acid in dichloromethane at 20; for 2 h;
Stage #2: with sodium tris(acetoxy)borohydride in dichloromethane at 20;
Steps:
Intermediate 3-f: 3-nitro-4- ((1- (oxetan-3-yl) piperidin-4-yl) amino) benzenesulfonamide Step 1: tert-butyl (1- (oxetan-3-yl) piperidin-4-yl) carbamate
To a solution of tert-butyl piperidin-4-ylcarbamate (1 g, 5 mmol) in DCM (50 ml) was added oxetan-3-one (1.08 g, 15 mmol), HOAC (0.2 ml). The mixture was stirred at room temperature for 2 hours. Then to the mixture was added NaBH (OAc) 3 (3.18 g, 15 mmol). The mixture was stirred at r.t. for overnight. The mixture was diluted with DCM (200 ml), washed with saturated aq. NaHCO 3 (100 ml), brine (200 mlx2), dried over Na 2SO 4, concentrated. The reaction residue was purified by chromatography column on silica (MeOH/DCM = 1/20) to give the product (1 g, 78%) as a yellow oil. 1H NMR (400 MHz, DMSO-d 6) δ ppm: 6.79 (d, J =6.3 Hz, 1H), 4.59-4.24 (m, 4H), 3.32-3.26 (m, 1H), 3.25-3.07 (m, 1H), 2.72-2.50 (m, 3H), 1.84-1.55 (m, 4H), 1.47-1.13 (m, 11H). MS (ESI, m/e) [M+1] + 257.1.
References:
WO2019/210828,2019,A1 Location in patent:Paragraph 0565; 0566; 0567