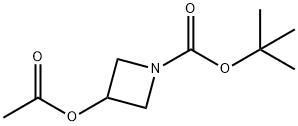
tert-Butyl 3-acetoxyazetidine-1-carboxylate synthesis
- Product Name:tert-Butyl 3-acetoxyazetidine-1-carboxylate
- CAS Number:1215205-53-0
- Molecular formula:C10H17NO4
- Molecular Weight:215.25

108-24-7
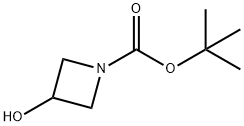
141699-55-0

1215205-53-0
General procedure for the synthesis of tert-butyl 3-acetoxyazetidine-1-carboxylate from ethanoic anhydride and N-Boc-3-hydroxyazetidine: 0.27 g (1.559 mmol) of N-Boc-3-hydroxyazetidine, 0.61 g (4.677 mmol) of diisopropylethylamine and a catalytic amount of 4-dimethylaminopyridine were dissolved at 0 °C in 7 mL of dichloromethane in dichloromethane. To this solution 0.32 g (2 mmol) of acetic anhydride was slowly added, followed by stirring the reaction for 16 hours at room temperature. After completion of the reaction, the solvent was removed by vacuum distillation. The crude product was purified by column chromatography using a mixed solvent system of hexane and ethyl acetate (3:1, v/v) to give 0.33 g (98% yield) of tert-butyl 3-acetoxyazetidine-1-carboxylate. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 5.12 (1H, m), 4.23 (2H, dd), 4.12 (2H, dd), 2.09 (3H, s), 1.44 (9H, s).

108-24-7
0 suppliers
$14.00/250ML

141699-55-0
396 suppliers
$6.00/1g

1215205-53-0
24 suppliers
inquiry
Yield:1215205-53-0 98%
Reaction Conditions:
with dmap;N-ethyl-N,N-diisopropylamine in dichloromethane at 0 - 20; for 16 h;
Steps:
1-84-1
Preparation Example 1-84-13-Acetoxy-azetidine-1-carboxylic acid tert-butyl ester 0.27 g (1.559 mmol) of the compound obtained from Preparation Example 1-83-2, 0.61 g (4.677 mmol) of diisopropylethylamine and a catalytic amount of 4-dimethylaminopyridine were dissolved in 7 mL of dichloromethane and cooled to 0° C. To the solution was added 0.32 g (2 mmol) of acetic anhydride and stirred at room temperature for 16 hours. The reaction mixture was distilled in vacuo to remove a solvent and purified by column chromatography using a mixed solution of hexane and ethyl acetate in the ratio of 3:1 to obtain the title compound 0.33 g (98%).1H NMR (400 MHz, CDCl3); δ 5.12 (1H, m), 4.23 (2H, dd), 4.12 (2H, dd), 2.09 (3H, s), 1.44 (9H, s)
References:
US2011/166121,2011,A1 Location in patent:Page/Page column 56

141699-55-0
396 suppliers
$6.00/1g

1215205-53-0
24 suppliers
inquiry
127-08-2
670 suppliers
$6.00/25g
254454-54-1
265 suppliers
$15.00/1g

1215205-53-0
24 suppliers
inquiry

24424-99-5
867 suppliers
$13.50/25G

1215205-53-0
24 suppliers
inquiry